Abstract
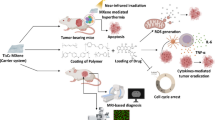
Cancer is the deadliest disease that has affected human health all over the world. Despite the much research that has been done in this field so far, there is an urgent need for improved techniques for diagnosis and treatment of cancer. In recent years, innovative nanomaterial-assisted therapies and diagnostics have led to significant enhancements in cancer treatment. In this context, two-dimensional nanomaterials (2DNMs) and their nanocomposites have been explored for cancer diagnostics and therapy due to their unique shape, size, chemical composition, biodegradability, and biocompatibility. The studies indicate that 2DNMs have significant potential in biomedicine, particularly in multimodal imaging, biosensors, drug/gene delivery, and cancer therapy. Due to high specific surface area, 2DNMs can efficiently adsorb molecules via covalent or non-covalent interactions and usage as carriers in controlled release systems in response to external stimuli. In addition, unique sheet-like nanostructures and photothermal converting ability led to 2DNMs being promising candidates for optical therapies such as photothermal therapy (PTT), photodynamic therapy (PDT), and theranostic. Furthermore, integrating 2DNMs in nanocomposites with further functional moieties become a new class of therapeutic agents in biomedicine substantially improving their features for synergistic cancer diagnosis and therapy. This chapter highlights the current state and benefits of using 2DNMs in cancer diagnosis and therapy and discusses the obstacles and prospects of their future development. Then we focus on 2DNMs applications in cancer treatment including smart drug delivery systems, PTT, and PDT. Lastly, the 2DNMs biocompatibility is also discussed to provide a unique overview of the topic.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abedi, M., Abolmaali, S.S., Heidari, R., Samani S.M.,Tamaddon, A.M.: Hierarchical mesoporous zinc-imidazole dicarboxylic acid MOFs: surfactant-directed synthesis, pH-responsive degradation, and drug delivery. Int. J. Pharm. 602,120685 (2021)
Alimardani, V., Abolmaali, S.S., Tamaddon, A.M., Ashfaq, M.: Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. Transl. Res. 11, 788–816 (2021)
Alipour, S., Shirazi, H.C., Kazemi, M., Dehshahri, A., Ahmadi, F.: Synthesis and cytotoxicity evaluation of doxorubicin-polyethyleneimine conjugate as a potential carrier for dual delivery of drug and gene. J. Drug Deliv. Sci. Technol. 68,102994 (2022)
Alimardani, V., Farahavar, G., Salehi, S., Taghizadeh, S., Ghiasi, M.R., Abolmaali, S.S.: Gold nanocages in cancer diagnosis, therapy, and theranostics: a brief review. Front. Mater. Sci. 15, 494–511 (2021)
Taghizadeh, S., Alimardani, V., Roudbali, P.L., Ghasemi, Y., Kaviani, E.: Gold nanoparticles application in liver cancer. Photodiagnosis Photodyn. Ther. 25, 389–400 (2019)
Alimardani, V., et al.: Nanotechnology-based cell-mediated delivery systems for cancer therapy and diagnosis. Drug Deliv. Transl. Res. 13, 189–221 (2023)
Chen, W., et al.: Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv. Mater. (Deerfield Beach, Fla) 29 (2017)
Peng, L., et al.: Monolayer nanosheets with an extremely high drug loading toward controlled delivery and cancer. Theranostics Adv. Mater. (Deerfield Beach, Fla) 30, e1707389 (2018)
Alimardani, V, Abolmaali, S.S., Borandeh, S.: Antifungal and antibacterial properties of graphene-based nanomaterials: a mini-review. J. Nanostructures 9, 402–413 (2019)
Borandeh, S., Alimardani, V., Abolmaali, S.S., Seppala, J.: Graphene family nanomaterials in ocular applications: physicochemical properties and toxicity. Chem. Res. Toxicol. 34, 1386–1402 (2021)
Novoselov, K.S., et al.: Electric field effect in atomically thin carbon films science 306, 666–669 (2004)
Ashfaq, M., Talreja, N., Chauhan, D., Afreen, S., Sultana, A., Srituravanich, W.: Two-dimensional (2D) hybrid nanomaterials for diagnosis and treatment of cancer. J. Drug Deliv. Sci. Technol. 103268 (2022)
Chen, Y., et al.: Two-dimensional metal nanomaterials: synthesis. Prop., Appl. Chem. Rev. 118, 6409–6455 (2018)
Wang, X., Cheng, L.: Multifunctional two-dimensional nanocomposites for photothermal-based combined cancer therapy. Nanoscale 11, 15685–15708 (2019)
Zhang, H., Fan, T., Chen, W., Li, Y., Wang, B.: Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact. Mater. 5, 1071–1086 (2020)
Gong, L., Yan, L., Zhou, R., Xie, J., Wu, W., Gu, Z.: Two-dimensional transition metal dichalcogenide nanomaterials for combination cancer therapy. J. Mater. Chem. B 5, 1873–1895 (2017)
Luo, S., et al.: Multifunctional photosensitizer grafted on polyethylene glycol and polyethylenimine dual-functionalized nanographene oxide for cancer-targeted near-infrared imaging and synergistic phototherapy. ACS Appl. Mater. Interfaces 8, 17176–17186 (2016)
Yang, G., et al.: Manganese dioxide coated WS2@ Fe3O4/sSiO2 nanocomposites for pH‐responsive MR imaging and oxygen‐elevated synergetic therapy. Small 14, 1702664 (2018)
Li, H., Fan, R., Zou, B., Yan, J., Shi, Q., Guo, G.: Roles of MXenes in biomedical applications: recent developments and prospects. Journal of Nanobiotechnology 21, 1–39 (2023)
Hong, X., Tan, C., Chen, J., Xu, Z., Zhang, H.: Synthesis, properties and applications of one-and two-dimensional gold nanostructures. Nano Res. 8, 40–55 (2015)
Nosheen, F., et al.: Ultrathin Pd-based nanosheets: syntheses, properties and applications. Nanoscale 12, 4219–4237 (2020)
Mishra, G., Dash, B., Pandey, S.: Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 153, 172–186 (2018)
Fan, J., Sun, M.: Transition metal dichalcogenides (tmdcs) heterostructures: synthesis, excitons and photoelectric properties. Chem. Rec. 22, e202100313 (2022)
Tamang, S., Rai, S., Bhujel, R., Bhattacharyya, N.K., Swain, B.P., Biswas, J.: A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloy. Compd. 169588 (2023)
Tian, Y., et al.: Inorganic boron-based nanostructures: synthesis, optoelectronic properties, and prospective applications. Nanomaterials 9, 538 (2019)
Luo, M., Fan, T., Zhou, Y., Zhang, H., Mei, L.: 2D black phosphorus–based biomedical applications. Adv. Funct. Mater. 29, 1808306 (2019)
Qin, Y., Wan, Y., Guo, J., Zhao, M.: Two-dimensional metal-organic framework nanosheet composites: preparations and applications. Chin. Chem. Lett. 33, 693–702 (2022)
Fang, Y., Liu, Y., Qi, L., Xue, Y., Li, Y.: 2D graphdiyne: an emerging carbon material. Chem. Soc. Rev. (2022)
Soltani, A., Faramarzi, M., Farjadian, F., Parsa, S.A.M., Panahi, H.A.: pH-responsive glycodendrimer as a new active targeting agent for doxorubicin delivery. Int. J. Biol. Macromol. 221, 508–522 (2022)
Farjadian, F., Ghasemi, S., Akbarian, M., Hoseini-Ghahfarokhi, M., Moghoofei, M., Doroudian, M.: Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 10 (2022)
Chakraborty, D., Ghosh, D., Kumar, S., Jenkins, D., Chandrasekaran, N., Mukherjee, A.: Nano-diagnostics as an emerging platform for oral cancer detection: Current and emerging trends. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 15, e1830 (2023)
Wen, W., et al.: Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 21, 164–177 (2018)
Wang, L., Xiong, Q., Xiao, F., Duan, H.: 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 89, 136–151 (2017)
Nawrot, W., Drzozga, K., Baluta, S., Cabaj, J., Malecha, K.: A fluorescent biosensors for detection vital body fluids’ agents. Sensors 18, 2357 (2018)
Son, M.H., Park, S.W., Sagong, H.Y., Jung, Y.K.: Recent advances in electrochemical and optical biosensors for cancer biomarker detection. BioChip J. 17, 44–67 (2023)
Mohammadpour-Haratbar, A., Boraei, S.B.A., Zare, Y., Rhee, K.Y., Park, S.-J.: Graphene-based electrochemical biosensors for breast cancer detection. Biosensors 13, 80 (2023)
He, Y., Lin, Y., Tang, H., Pang, D.: A graphene oxide-based fluorescent aptasensor for the turn-on detection of epithelial tumor marker mucin 1 Nanoscale 4, 2054–2059 (2012)
Kadhim, M.M., Rheima, A.M., Abbas, Z.S., Jlood, H.H., Hachim, S.K., Kadhum, W.R.: Evaluation of a biosensor-based graphene oxide-DNA nanohybrid for lung cancer RSC. Advances 13, 2487–2500 (2023)
Hu, Y., et al.: Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 1, 24–36 (2017)
Presutti, D., et al.: Transition metal dichalcogenides (TMDC)-based nanozymes for biosensing and therapeutic applications. Materials 15, 337 (2022)
Cai, B., Guo, S., Li, Y.: MoS 2-based sensor for the detection of miRNA in serum samples related to breast cancer. Anal. Methods 10, 230–236 (2018)
Sadighbayan, D., Hasanzadeh, M., Ghafar-Zadeh, E.: Biosensing based on field-effect transistors (FET): recent progress and challenges TrAC. Trends Anal. Chem. 133, 116067 (2020)
Syu, Y.-C., Hsu, W.-E., Lin, C.-T.: Field-effect transistor biosensing: Devices and clinical applications ECS. J. Solid State Sci. Technol. 7, Q3196 (2018)
Mao, S., Chang, J., Pu, H., Lu, G., He, Q., Zhang, H., Chen, J.: Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 46, 6872–6904 (2017)
Majd, S.M., Salimi, A., Ghasemi, F.: An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosens. Bioelectron. 105, 6–13 (2018)
Cai, B., Xia, Z., Wang, J., Wu, S., Jin, X.: Reduced graphene oxide-based field effect transistor biosensors for high-sensitivity miRNA21 detection. ACS Appl. Nano Mater. 5, 12035–12044 (2022)
Aziz, A., et al.: Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides. A review Microchimica Acta 186, 671 (2019)
Khatri, R., Puri, N.K.: Electrochemical studies of biofunctionalized MoS 2 matrix for highly stable immobilization of antibodies and detection of lung cancer protein biomarker. New J. Chem. 46, 7477–7489 (2022)
Jafari-Kashi, A., Rafiee-Pour, H.-A., Shabani-Nooshabadi, M.: A new strategy to design label-free electrochemical biosensor for ultrasensitive diagnosis of CYFRA 21–1 as a biomarker for detection of non-small cell lung cancer. Chemosphere 301, 134636 (2022)
Zhao, L., et al.: A fluorescent biosensor based on molybdenum disulfide nanosheets and protein aptamer for sensitive detection of carcinoembryonic antigen. Sens. Actuators, B Chem. 273, 185–190 (2018)
Oudeng, G., Au, M., Shi, J., Wen, C., Yang, M.: One-step in situ detection of miRNA-21 expression in single cancer cells based on biofunctionalized MoS2 Nanosheets. ACS Appl. Mater. Interfaces 10, 350–360 (2018)
Guo, S., Yang, F., Zhang, Y., Ning, Y., Yao, Q., Zhang, G.-J.: Amplified fluorescence sensing of miRNA by combination of graphene oxide with duplex-specific nuclease. Anal. Methods 6, 3598–3603 (2014)
Cai, B., Wang, S., Huang, L., Ning, Y., Zhang, Z., Zhang, G.J.: Ultrasensitive label-free detection of PNA-DNA hybridization by reduced graphene oxide field-effect transistor biosensor. ACS Nano 8, 2632–2638 (2014)
Lee, S.X., Lim, H.N., Ibrahim, I., Jamil, A., Pandikumar, A., Huang, N.M.: Horseradish peroxidase-labeled silver/reduced graphene oxide thin film-modified screen-printed electrode for detection of carcinoembryonic antigen. Biosens. Bioelectron. 89, 673–680 (2017)
Liu, D., Yang, F., Xiong, F., Gu, N.: The smart drug delivery system and its clinical potential. Theranostics 6, 1306 (2016)
Alimardani, V., Sadat Abolmaali, S., Yousefi, G., Hossein Nowroozzadeh, M., Mohammad Tamaddon, A.: In-situ nanomicelle forming microneedles of poly NIPAAm-b-poly glutamic acid for trans-scleral delivery of dexamethasone. J. Ind. Eng. Chem. 119, 485–498 (2023)
Ghasemi, S., Ahmadi, L., Farjadian, F.: Thermo-responsive PNIPAAm-b-PLA amphiphilic block copolymer micelle as nanoplatform for docetaxel drug release. J. Mater. Sci. 57, 17433–17447 (2022)
Ahmadi, S., et al.: Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today 34, 100914 (2020)
Farjadian, F., Ghasemi, S., Andami, Z., Tamami, B.: Thermo-responsive nanocarrier based on poly (N-isopropylacrylamide) serving as a smart doxorubicin delivery system. Iran. Polym. J. 29, 197–207 (2020)
Chu, Z., Liu, J., Guo, Z., Zhang, H.: 2 μm passively Q-switched laser based on black phosphorus. Opt. Mater. Express 6, 2374–2379 (2016)
Fan, T., Zhou, Y., Qiu, M., Zhang, H.: Black phosphorus: a novel nanoplatform with potential in the field of bio-photonic nanomedicine. J. Innov. Opt. Health Sci. 11, 1830003 (2018)
Zhao, Y., et al.: Stable and multifunctional dye-modified black phosphorus nanosheets for near-infrared imaging-guided photothermal therapy. Chem. Mater. 29, 7131–7139 (2017)
Liu, N., Fan, F., Xu, W., Zhang, H., Zhou, Q., Li, X.: Synthesis of functional hollow WS2 particles with large surface area for Near-Infrared (NIR) triggered drug delivery. J. Alloy. Compd. 875, 160034 (2021)
Farzanfar, J., Farjadian, F., Roointan, A., Mohammadi-Samani, S., Tayebi, L.: Assessment of pH responsive delivery of methotrexate based on PHEMA-st-PEG-DA nanohydrogels. Macromol. Res. 29, 54–61 (2021)
Sawant, R.M., Hurley, J., Salmaso, S., Kale, A., Tolcheva, E., Levchenko, T., Torchilin, V.: “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chem. 17, 943–949 (2006)
Lee, G.-Y., Lo, P.-Y., Cho, E.-C., Zheng, J.-H., Li, M., Huang, J.-H., Lee, K.-C.: Integration of PEG and PEI with graphene quantum dots to fabricate pH-responsive nanostars for colon cancer suppression in vitro and in vivo. FlatChem 31, 100320 (2022)
Jung, H.S., Verwilst, P., Sharma, A., Shin, J., Sessler, J.L., Kim, J.S.: Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem. Soc. Rev. 47, 2280–2297 (2018)
Melamed, J.R., Edelstein, R.S., Day, E.S.: Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 9, 6–11 (2015)
Lin, C., Hao, H., Mei, L., Wu, M.: Metal-free two-dimensional nanomaterial-mediated photothermal tumor therapy. Smart Mater. Med. 1, 150–167 (2020)
Qin, Z., Bischof, J.C.: Thermophysical and biological responses of gold nanoparticle laser heating. Chem. Soc. Rev. 41, 1191–1217 (2012)
Kim, H., Lee, D., Kim, J., Kim, T.-I., Kim, W.J.: Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 7, 6735–6746 (2013)
Liu, Y., Bhattarai, P., Dai, Z., Chen, X.: Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48, 2053–2108 (2019)
Yan, M., et al.: Nanoscale reduced graphene oxide-mediated photothermal therapy together with IDO inhibition and PD-L1 blockade synergistically promote antitumor immunity ACS Appl. Mater. Interfaces 11, 1876–1885 (2018)
Yan, M., et al.: nanoscale reduced graphene oxide-mediated photothermal therapy together with IDO inhibition and PD-L1 blockade synergistically promote antitumor immunity. ACS Appl. Mater. Interfaces 11, 1876–1885 (2019)
Spikes, J.D., Bommer, J.C.: Photosensitizing properties of mono-l-aspartyl chlorin e6 (NPe6): a candidate sensitizer for the photodynamic therapy of tumors. J. Photochem. Photobiol. B: Biol. 17, 135–143 (1993)
Master, A., Livingston, M., Sen Gupta, A.: Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J. Control. Release 168, 88–102 (2013)
Gazzi, A., et al.: Photodynamic therapy based on graphene and MXene in cancer theranostics. Front. Bioeng. Biotechnol. 7 (2019)
Sahu, A., Choi, W.I., Lee, J.H., Tae, G.: Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 34, 6239–6248 (2013)
Tian, B., Wang, C., Zhang, S., Feng, L., Liu, Z.: Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 5, 7000–7009 (2011)
Yang, G., et al.: Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 18, 2475–2484 (2018)
Wang, H., et al.: SDS coated Fe3O4@MoS2 with NIR-enhanced photothermal-photodynamic therapy and antibiotic resistance gene dissemination inhibition functions. Colloids Surf.S B: Biointerfaces 214,112457 (2022)
Cao, Y., et al.: A near-infrared triggered upconversion/MoS2 nanoplatform for tumour-targeted chemo-photodynamic combination therapy colloids and Surfaces B: Biointerfaces 213, 112393 (2022)
Avitabile, E., Bedognetti, D., Ciofani, G., Bianco, A., Delogu, L.G.: How can nanotechnology help the fight against breast cancer? Nanoscale 10, 11719–11731 (2018)
Viseu, T., Lopes, C.M., Fernandes, E., Real Oliveira, M.E.C., Lucio, M.: A systematic review and critical analysis of the role of graphene-based nanomaterials in cancer theranostics. Pharmaceutics 10, 282 (2018)
Gollavelli, G., Ling, Y.-C.: Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 35, 4499–4507 (2014)
Gulzar, A., Xu, J., Yang, D., Xu, L., He, F., Gai, S., Yang, P.: Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 47, 3931–3939 (2018)
Wu, C., Zhu, A., Li, D., Wang, L., Yang, H., Zeng, H., Liu, Y.: Photosensitizer-assembled PEGylated graphene-copper sulfide nanohybrids as a synergistic near-infrared phototherapeutic agent. Expert Opin. Drug Deliv. 13, 155–165 (2016)
Yang, K., Feng, L., Hong, H., Cai, W., Liu, Z.: Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 8, 2392–2403 (2013)
Huang, P., et al.: Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics 1, 240 (2011)
Zhou, L., Jiang, H., Wei, S., Ge, X., Zhou, J., Shen, J.: High-efficiency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon 50, 5594–5604 (2012)
Zhou, L., et al.: Combination of chemotherapy and photodynamic therapy using graphene oxide as drug delivery system. J. Photochem. Photobiol. B. 135, 7–16 (2014)
Zhang, Q., et al.: The theranostic nanoagent Mo2C for multi-modal imaging-guided cancer synergistic phototherapy. Biomater. Sci. 7, 2729–2739 (2019)
Liu, G., et al.: Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy ACS. Appl. Mater. Interfaces 9, 40077–40086 (2017)
Liu, T., et al.: Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale 6, 11219–11225 (2014)
Peng, M.Y., Zheng, D.W., Wang, S.B., Cheng, S.X., Zhang, X.Z.: Multifunctional nanosystem for synergistic tumor therapy delivered by two-dimensional MoS(2). ACS Appl. Mater Interfaces 9, 13965–13975 (2017)
Li, S., et al.: Enhanced photothermal-photodynamic therapy by indocyanine green and curcumin-loaded layered MoS(2) Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomedicine 16, 433–442 (2021)
Song, C., et al.: MoS2-Based multipurpose theranostic nanoplatform: realizing dual-imaging-guided combination phototherapy to eliminate solid tumor via a liquefaction necrosis process. J. Mater. Chem. B 5, 9015–9024 (2017)
Yougbaré, S., et al.: Gold nanorod-decorated metallic MoS2 nanosheets for synergistic photothermal and photodynamic antibacterial therapy. Nanomaterials 11, 3064 (2021)
Yang, L., Wang, J., Yang, S., Lu, Q., Li, P., Li, N.: Rod-shape MSN@MoS(2) Nanoplatform for FL/MSOT/CT imaging-guided photothermal and photodynamic therapy. Theranostics 9, 3992–4005 (2019)
Yang, G., Gong, H., Liu, T., Sun, X., Cheng, L., Liu, Z.: Two-dimensional magnetic WS2@Fe3O4 nanocomposite with mesoporous silica coating for drug delivery and imaging-guided therapy of cancer. Biomaterials 60, 62–71 (2015)
Yong, Y, et al.: WS2 nanosheet as a new photosensitizer carrier for combined photodynamic and photothermal therapy of cancer cells. Nanoscale 6, 10394–10403 (2014)
Liao, W., Zhang, L., Zhong, Y., Shen, Y., Li, C., An, N.: Fabrication of ultrasmall WS2 quantum dots-coated periodic mesoporous organosilica nanoparticles for intracellular drug delivery and synergistic chemo-photothermal therapy. OncoTargets Ther. 11, 1949–1960 (2018)
Yu, C., et al.: Ti3C2Tx MXene loaded with indocyanine green for synergistic photothermal and photodynamic therapy for drug-resistant bacterium. Colloids Surf. B. Biointerfaces 217, 112663 (2022)
Ahirwar, S., Mallick, S., Bahadur, D.: Photodynamic therapy using graphene quantum dot derivatives. J. Solid State Chem. 282, 121107 (2020)
Guo, W., et al.: Graphene oxide (GO)-based nanosheets with combined chemo/photothermal/photodynamic therapy to overcome gastric cancer (GC) paclitaxel resistance by reducing mitochondria-derived adenosine-triphosphate (ATP). J. Nanobiotechnology 19, 146 (2021)
Ashkarran, A.A., Swann, J., Hollis, L., Mahmoudi, M.: The file drawer problem in nanomedicine. Trends Biotechnol. 39, 425–427 (2021)
Liu, Y., Zhu, S., Gu, Z., Chen, C., Zhao, Y.: Toxicity of manufactured nanomaterials. Particuology 69, 31–48 (2022)
Derakhshi, M., Daemi, S., Shahini, P., Habibzadeh, A., Mostafavi, E., Ashkarran, A.A.: Two-Dimensional Nanomaterials beyond graphene for biomedical applications. J. Funct. Biomater. 13 (2022)
Duch, M.C., et al.: Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 11, 5201–5207 (2011)
Bussy, C., Ali-Boucetta, H., Kostarelos, K.: Safety considerations for graphene: lessons learnt from carbon nanotubes. Acc. Chem. Res. 46, 692–701 (2013)
Jayakumar, A., Surendranath, A., Mohanan, P.: 2D materials for next generation healthcare applications. Int. J. Pharm. 551, 309–321 (2018)
Li, M., Luo, Z., Zhao, Y.: Recent advancements in 2D nanomaterials for cancer therapy. Sci. China Chem. 61, 1214–1226 (2018)
Lin, H., Chen, Y., Shi, J.: Insights into 2D MXenes for versatile biomedical applications: current advances and challenges ahead. Adv. Sci. 5, 1800518 (2018)
Kyriakides, T.R., et al.: Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 16 (2021)
Bullock, C.J., Bussy, C.: Biocompatibility considerations in the design of graphene biomedical materials advanced materials. Interfaces 6, 1900229 (2019)
Liao, C., Li, Y., Tjong, S.C.: Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 19 (2018).
Maio, A., Pibiri, I., Morreale, M., Mantia, F.P.L., Scaffaro, R.: An overview of functionalized graphene nanomaterials for advanced. Appl. Nanomater. 11, 1717 (2021)
Jones, C.F., Grainger, D.W.: In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 61, 438–456 (2009)
Liao, C., Li, Y., Tjong, S.C.: Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 19, 3564 (2018)
Ou, L., et al.: Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part. Fibre Toxicol. 13, 57 (2016)
Zhang, B., Wei, P., Zhou, Z., Wei, T.: Interactions of graphene with mammalian cells: molecular mechanisms and biomedical insights. Adv. Drug Deliv. Rev. 105, 145–162 (2016)
Duan, G., et al.: Graphene-induced pore formation on cell membranes. Sci. Rep. 7, 42767 (2017)
Bach, D., Wachtel, E.: Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochimica et Biophysica Acta (BBA) - Biomembranes 1610, 187–197 (2003)
Ermilova, I., Lyubartsev, A.P.: Cholesterol in phospholipid bilayers: positions and orientations inside membranes with different unsaturation degrees. Soft Matter 15, 78–93 (2019)
Zhan, J., Lei, Z., Zhang, Y.: Non-covalent interactions of graphene surface: mechanisms and applications. Chem 8, 947–979 (2022)
Li, S., Stein, A.J., Kruger, A., Leblanc, R.M.: Head groups of lipids govern the interaction and orientation between graphene oxide and lipids. J. Phys. Chem. C 117, 16150–16158 (2013)
Hu, X., Lei, H., Zhang, X., Zhang, Y.: Strong hydrophobic interaction between graphene oxide and supported lipid bilayers revealed by AFM. Microsc. Res. Tech. 79, 721–726 (2016)
Li, R.: et al.: Surface oxidation of graphene oxide determines membrane damage, lipid peroxidation, and cytotoxicity in macrophages in a pulmonary toxicity model. ACS Nano 12, 1390–1402 (2018)
Pelin, M. et al.: Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: the role of xanthine oxidase and NADH dehydrogenase. Nanoscale 10, 11820–11830 (2018)
Liu, J.H., Yang, S.T., Wang, H., Chang, Y., Cao, A., Liu, Y.: Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine (Lond.) 7, 1801–1812 (2012)
Kurantowicz, N., et al.: Biodistribution of a high dose of diamond, graphite, and graphene oxide nanoparticles after multiple intraperitoneal injections in rats. Nanoscale Res. Lett. 10, 398 (2015)
Strojny, B., et al.: Long term influence of carbon nanoparticles on health and liver status in rats. PloS one 10, e0144821 (2015)
Baur, X., Sanyal, S., Abraham, J.L.: Mixed-dust pneumoconiosis: review of diagnostic and classification problems with presentation of a work-related case. Sci. Total. Environ. 652, 413–421 (2019)
Lin, H., Gao, S., Dai, C., Chen, Y., Shi, J.: Correction to A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 142, 10567–10567 (2020)
Lin, H., Wang, X., Yu, L., Chen, Y., Shi, J.: Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 17, 384–391 (2017)
Lin, H., Wang, Y., Gao, S., Chen, Y., Shi, J.: Theranostic 2D tantalum carbide (MXene). Adv. Mater. 30, 1703284 (2018)
Yang, C., Xu, D., Peng, W., Li, Y., Zhang, G., Zhang, F., Fan, X.: Ti2C3Tx nanosheets as photothermal agents for near-infrared responsive hydrogels. Nanoscale 10, 15387–15392 (2018)
Dai, C. Lin, H., Xu, G., Liu, Z., Wu, R., Chen, Y.: Biocompatible 2D titanium carbide (MXenes) composite nanosheets for pH-responsive MRI-guided tumor hyperthermia. Chem. Mater. 29, 8637–8652 (2017)
Xing, C., et al.: Two-dimensional MXene (Ti3C2)-integrated cellulose hydrogels: toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces 10, 27631–27643 (2018)
Pandey, R.P., Rasool, K., Madhavan, V.E., Aïssa, B., Gogotsi, Y., Mahmoud, K.A.: Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 6, 3522–3533 (2018)
Rakhi, R., Nayak, P., Xia, C., Alshareef, H.N.: Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 6, 1–10 (2016)
Wu, L., et al.: 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 107, 69–75 (2018)
Lim, G.P., Soon, C.F., Ma, N.L., Morsin, M., Nayan, N., Ahmad, M.K., Tee, K.S.: Cytotoxicity of MXene-based nanomaterials for biomedical applications: A mini review. Environ. Res. 201, 111592 (2021)
Arabi Shamsabadi, A., Sharifian Gh, M., Anasori, B., Soroush, M.: Antimicrobial mode-of-action of colloidal Ti3C2Tx MXene nanosheets. ACS Sustain. Chem. Eng. 6, 16586–16596 (2018)
Jastrzębska, A.M., et al.: On the rapid in situ oxidation of two-dimensional V2CTz MXene in culture cell media and their cytotoxicity. Mater. Sci. Eng. C 119, 111431 (2021)
Jastrzębska, A.M., et al.: On tuning the cytotoxicity of Ti3C2 (MXene) flakes to cancerous and benign cells by post-delamination surface modifications 2D. Materials 7, 025018 (2020)
Liu, J., Pope, C.N.: Chapter 21 - Intrinsic and extrinsic factors that can modify toxicity. In: Pope, C.N., Liu, J. (eds.). An Introduction to Interdisciplinary Toxicology, pp. 285–293. Academic Press (2020). https://doi.org/10.1016/B978-0-12-813602-7.00021-1
Jastrzębska, A.M., et al.: In vitro studies on cytotoxicity of delaminated Ti3C2 MXene. J. Hazard. Mater. 339, 1–8 (2017)
Ganguly, P., Breen, A., Pillai, S.C.: Toxicity of nanomaterials: exposure, pathways, assessment, and recent advances ACS. Biomater. Sci. Eng. 4, 2237–2275 (2018)
Wu, W., Ge, H., Zhang, L., Lei, X., Yang, Y., Fu, Y., Feng, H.: Evaluating the Cytotoxicity of Ti3C2 MXene to neural stem. Cells Chem. Res. Toxicol. 33, 2953–2962 (2020)
Teo, W.Z., Chng, E.L.K., Sofer, Z., Pumera, M.: Cytotoxicity of exfoliated transition‐metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem. A Eur. J. 20, 9627–9632 (2014)
Wu, H., et al.: Biocompatible inorganic fullerene-like molybdenum disulfide nanoparticles produced by pulsed laser ablation in water. ACS Nano 5, 1276–1281 (2011)
Gupta, M., Gupta, S.: An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 7, 2074 (2017)
Pereira, B.B., Limongi, J.E., Campos Júnior, E.Od., Luiz, D.P., Kerr, W.E.: Effects of piperonyl butoxide on the toxicity of the organophosphate temephos and the role of esterases in the insecticide resistance of Aedes aegypti Revista da Sociedade Brasileira de Medicina Tropical 47, 579–582 (2014)
Saha, U., Fayiga, A., Hancock, D., Sonon, L.: Selenium in animal nutrition: deficiencies in soils and forages, requirements, supplementation and toxicity. Int. J. Appl. Agric. Sci. 2, 112–125 (2016)
Nurunnabi, M., McCarthy, J.: Biomedical applications of graphene and 2D nanomaterials. Elsevier (2019)
Moroder, L.: Isosteric replacement of sulfur with other chalcogens in peptides and proteins. J. Pept. Sci.: Off. Publ. Eur. Pept. Soc. 11, 187–214 (2005)
Nath, N.C.D., Debnath, T., Nurunnabi, M., Kim, E.-K.: In vitro toxicity of 2D materials. In: Biomedical Applications of Graphene and 2D Nanomaterials. Elsevier, pp 165–186 (2019)
Wang, X., et al.: Differences in the toxicological potential of 2D versus aggregated molybdenum disulfide in the lung. Small 11, 5079–5087 (2015)
Yin, W., et al.: High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 8, 6922–6933 (2014)
Chou, S.S., et al.: Ligand conjugation of chemically exfoliated MoS2. J. Am. Chem. Soc. 135, 4584–4587 (2013)
Kim, J.S., Yoo, H.W., Choi, H.O., Jung, H.T.: Tunable volatile organic compounds sensor by using thiolated ligand conjugation on MoS2. Nano Lett. 14, 5941–5947 (2014)
Anielle Christine Almeida, S., et al.: Biocompatibility of doped semiconductors nanocrystals and nanocomposites. In: Tülay Aşkin, Ç. (ed.) Cytotoxicity. IntechOpen, Rijeka, p Ch. 9. (2018). https://doi.org/10.5772/intechopen.77197
Dymek, M., Sikora, E.: Liposomes as biocompatible and smart delivery systems—the current state. Adv. Colloid Interface Sci. 309, 102757 (2022)
Saroia, J., Yanen, W., Wei, Q., Zhang, K., Lu, T., Zhang, B.: A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 1, 265–279 (2018)
Areecheewakul, S., et al.: Toxicity assessment of metal oxide nanomaterials using in vitro screening and murine acute inhalation studies. NanoImpact 18, 100214 (2020)
Park, E.-J., Lee, G.-H., Yoon, C., Kim, D.-W.: Comparison of distribution and toxicity following repeated oral dosing of different vanadium oxide nanoparticles in mice. Environ. Res. 150, 154–165 (2016)
Uche, F., Obianime, A., Gogo-Abite, M.: Effects of vanadium pentoxide on the histological and sperm parameters of male guinea pigs. Chem. A Eur. J. 12 (2008)
Morris, A.S., Givens, B.E., Silva, A., Salem, A.K.: Copper oxide nanoparticle diameter mediates serum‐sensitive toxicity in BEAS‐2B cells. Adv. NanoBiomed Res. 1, 2000062 (2021)
Naz, S., Gul, A., Zia, M.: Toxicity of copper oxide nanoparticles: a review study. IET Nanobiotechnol. 14, 1–13 (2020)
Worthington, K.L., et al.: Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology 24, 395101 (2013)
Sánchez-López, E., et al.: Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10, 292 (2020)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abedi, M., Asadi, M., Mehrzadeh, M., Rahiminezhad, Z., Ghasemi, Y., Alimardani, V. (2024). Two-Dimensional (2D)-Based Hybrid Composites for Cancer Diagnosis and Therapy. In: Talreja, N., Chauhan, D., Ashfaq, M. (eds) Two-dimensional Hybrid Composites. Engineering Materials. Springer, Singapore. https://doi.org/10.1007/978-981-99-8010-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-99-8010-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8009-3
Online ISBN: 978-981-99-8010-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)