Abstract
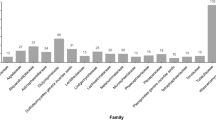
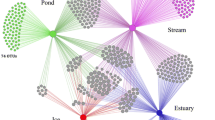
The biodiversity of fungi in freshwater habitats is very high, and their ecological roles are significant. By estimation, there may be more than 3000 species including ascomycetes, basidiomycetes, chytrids, and fungal-like waterborne oomycetes. They are distributed globally as saprobes, animal pathogens, and even plankton parasites. New species have been reported repeatedly through ecological and taxonomical studies based on morphologies and recently through molecular barcodes utilizing latest sequencing technologies. Herein we review their biology and ecological role in the environment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdel-Aziz FA (2008) Diversity of aquatic fungi on Phragmites australis at Lake Manzala, Egypt. Sydowia 60(1):1–14, PubMed PMID: WOS:000257909100001
Abdel-Raheem AM, Ali EH (2004) Lignocellulolytic enzyme production by aquatic hyphomycetes species isolated from the Nile’s delta region. Mycopathologia 157:277–286
Abdel-Raheem A, Shearer C (2002) Extracellular enzyme production by freshwater ascomycetes. Fungal Divers 11:1–19
Aimer RD, Segedin BP (1985) Some aquatic hyphomycetes from New Zealand streams. N Z J Bot 23:273–299
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Evol 215:403–410
Anderson JL, Shearer CA (2011) Population genetics of the aquatic fungus Tetracladium marchalianum over space and time. PLoS One 6(1):10. doi:10.1371/journal.pone.0015908, PubMed PMID: WOS:000286516500015
Ando K (1992) A study of terrestrial aquatic hyphomycetes. Trans Mycol Soc Jpn 33:415–425
Ando K, Tubaki K (1984a) Some undescribed hyphomycetes in rainwater draining from intact trees. Trans Mycol Soc Jpn 25:39–47
Ando K, Tubaki K (1984b) Some undescribed hyphomycetes in the rain drops from intact leaf-surface. Trans Mycol Soc Jpn 25:21–37
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88:541–549
Au DWT, Jones EBG, Moss ST (1996) Spore attachment and extracellular mucilage of aquatic hyphomycetes. Biofouling 10:123–140
Bärlocher F (1992) The ecology of aquatic hyphomycetes. Springer, Berlin
Bärlocher F (2010) Molecular approaches promise a deeper and broader understanding of the evolutionary ecology of aquatic hyphomycetes. J N Am Benthol Soc 29(3):1027–1041. doi:10.1899/09-081.1, PubMed PMID: WOS:000280692400021
Bärlocher F, Kendrick B (1974) Dynamics of the fungal population on leaves in a stream. J Ecol 62:761–791
Bärlocher F, Seena S, Wilson KP, Williams DD (2008) Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw Biol 53(2):368–379. doi:10.1111/j.1365-2427.2007.01899.x, PubMed PMID: WOS:000252393800013
Baschien C, Marvanová L, Szewzyk U (2006) Phylogeny of selected aquatic hyphomycetes based on morphological and molecular data. Nova Hedwigia 83(3–4):311–352. doi:10.1127/0029-5035/2006/0083-0311, PubMed PMID: WOS:000242664300003
Baschien C, Rode G, Böckelmann U, Götz F, Szewzyk U (2009) Interactions between hyphosphere-associated bacteria and the fungus Cladosporium herbarum on aquatic leaf litter. Microb Ecol 58(3):642–650. doi:10.1007/s00248-009-9528-6, PubMed PMID: WOS:000269928300020
Baschien C, Tsui CKM, Gulis V, Szewzyk U, Marvanová L (2013) The molecular phylogeny of aquatic hyphomycetes with affinity to the Leotiomycetes. Fungal Biol 117(9):660–672. doi:10.1016/j.funbio.2013.07.004, PubMed PMID: WOS:000324899800008
Beakes GW (2003) Lower fungi: a review of microscopial techniques for the taxonomic and ecological study of zoosporic freshwater fungi. In: Tsui CKM, Hyde KD (eds) Fungal diversity research series 10 freshwater mycology -a practical approach. Fungal Diversity Press, Hong Kong, pp 51–79
Belliveau MJ-R, Bärlocher F (2005) Molecular evidence confirms multiple origins of aquatic hyphomycetes. Mycol Res 109(12):1407–1417, PubMed
Berbee ML, Taylor JW (2001) Fungal molecular evolution: gene trees and geologic time. In: Mc Laughlin DJ and Mc Laughlin EJ, Lemke PA (eds) The mycota VII part b systematics and evolution. Springer, Berlin
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL et al (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95(15):9031–9036. doi:10.1073/pnas.95.15.9031, PubMed PMID: WOS:000075143900110
Bermingham S, Fisher PJ, Martin A, Marriott M, Lappin-Scott H (1998) The effect of the herbicide Mecoprop on Heliscus lugdunensis and its influence on the preferential feeding of Gammarus pseudolimnaeus. Microb Ecol 35:199–204
Beverwijk AL (1951) Candelabrum spinulosum, a new fungus species. Anton Leeuw Int J Gen Mol Microbiol 17:9–12
Bowman BH, Taylor JW, Brown AG, Lee J, Lu SD, White TJ (1992) Molecular evolution of the fungi relationship of the basidiomycetes, ascomycetes and chytridiomycetes. Mol Biol Evol 9:285–296
Bucher VVC, Hyde KD, Pointing SB, Reddy CA (2004) Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers 15:1–14
Buczacki ST (1983) Zoosporic plant pathogens: a modern perspective. Academic, London
Cai L, Zhang KQ, McKenzie EHC, Hyde KD (2003) New species of Dictyosporium and Digitodesmium from submerged wood in Yunnan, China. Sydowia 55(2):129–135, PubMed PMID: WOS:000188231200001
Campbell J, Shearer C, Marvanová L (2006) Evolutionary relationships among aquatic anamorphs and teleomorphs: Lemonniera, Margaritispora, and Goniopila. Mycol Res 110:1025–1033. doi:10.1016/j.mycres.2006.04.012, PubMed PMID: WOS:000241957700003
Campbell J, Marvanová L, Gulis V (2009) Evolutionary relationships between aquatic anamorphs and teleomorphs: Tricladium and Varicosporium. Mycol Res 113:1322–1334. doi:10.1016/j.mycres.2009.09.003, PubMed PMID: WOS:000272811000009
Casas JJ, Gessner MO, Lopez D, Descals E (2011) Leaf-litter colonisation and breakdown in relation to stream typology: insights from Mediterranean low-order streams. Freshw Biol 56(12):2594–2608. doi:10.1111/j.1365-2427.2011.02686.x, PubMed PMID: WOS:000296502000014
Chamier A (1985) Cell-wall-degrading enzymes of aquatic hyphomycetes: a review. Bot J Linn Soc 91:67–81
Chandrashekar KR, Kaveriappa KM (1991) Production of extracellular cellulase by Lunulospora curvula and Flagellospora penicillioides. Folia Microbiol 36:249–255
Chauvet E (1991) Aquatic hyphomycete distribution in South-Western France. J Biogeogr 18:699–706
Chukanhom K, Hatai K (2004) Freshwater fungi isolated from eggs of the common carp (Cyprinus carpio) in Thailand. Mycoscience 45:42–48
Clivot H, Cornut J, Chauvet E, Elger A, Poupin P, Guerold F et al (2014) Leaf-associated fungal diversity in acidified streams: insights from combining traditional and molecular approaches. Environ Microbiol 16(7):2145–2156. doi:10.1111/1462-2920.12245, PubMed PMID: WOS:000338983600013
Cole JJ, Caraco NF, Likens GE (1990) Short-range atmospheric transport—a significant source of phosphorus to an Oligotrophic Lake. Limnol Oceanogr 35(6):1230–1237, PubMed PMID: WOS:A1990EP94300002
Digby S, Goos RD (1987) Morphology, development and taxonomy of Loramyces. Mycologia 79:821–831
Dix NJ, Webster J (1995) Fungal ecology. Springer, Berlin
Doggett MS (2000) Characterization of fungal biofilms within a municipal water distribution system. Appl Environ Microbiol 66(3):1249–1251. doi:10.1128/aem.66.3.1249-1251.2000, PubMed PMID: WOS:000085604800059
Duarte S, Seena S, Baerlocher F, Cassio F, Pascoal C (2012) Preliminary insights into the phylogeography of six aquatic hyphomycete species. PLoS One 7(9), e45289. doi:10.1371/journal.pone.0045289, PubMed PMID: WOS:000311313900114
Duarte S, Baerlocher F, Cassio F, Pascoal C (2014) Current status of DNA barcoding of aquatic hyphomycetes. Sydowia 66(2):191–202, PubMed PMID: WOS:000347436300003
Eaton RA, Jones EBG (1971a) The biodeterioration of timber in water-cooling towers. II. Fungi growing on wood in different positions in a water cooling system. Mater Org 6:81–92
Eaton RA, Jones EGB (1971b) The biodeterioration of timber in water cooling towers. I. Fungal ecology and the decay of wood at Connah’s Quay and Ince. Mater Org 6:51–80
Eaton RA, Hale MDC (1993) Wood: decay, pests and protection. Chapman & Hall, London
Fallah PM, Shearer CA (2001) Freshwater ascomycetes: new or noteworthy species from north temperate lakes in Wisconsin. Mycologia 93(3):566–602. doi:10.2307/3761741, PubMed PMID: WOS:000168935100016
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graça MA (2014) A meta‐analysis of the effects of nutrient enrichment on litter decomposition in streams. Biol Rev. doi:10.1111/brv.12125
Ferrer A, Miller AN, Shearer CA (2011) Minutisphaera and Natipusilla: two new genera of freshwater Dothideomycetes. Mycologia 103(2):411–423. doi:10.3852/10-177, PubMed PMID: WOS:000288887400017
Fisher PJ, Sharma PD, Webster J (1977) Cellulolytic ability of aero-aquatic hyphomycetes. Trans Br Mycol Soc 69:495–496, PubMed PMID: WOS:A1977EF05300018
Fisher PJ, Petrini O, Webster J (1991) Aquatic hyphomycetes and other fungi in living aquatic and terrestrial roots of Alnus glutinosa. Mycol Res 95:543–547
Fuller MS, Jaworski A (1987) Zoosporic fungi in teaching and research. Southeastern Publishing Corporation, Athen, GA
Gessner MO, Gulis V, Kuehn KA, Chauvet E, Suberkropp K (2007) 17 Fungal decomposers of plant litter in aquatic ecosystems. In: Christian PK, Druzhinina IS (eds) Environmental and microbial relationships, 4th edn. Springer, Berlin
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH et al (2010) Diversity meets decomposition. Trends Ecol Evol 25(6):372–380, PubMed PMID: WOS:000278682500008
Goh TK (1997) Tropical freshwater hyphomycetes. In: Hyde KD (ed) Biodiversity of tropical microfungi. Hong Kong University Press, Hong Kong
Goh T, Hyde K (1996a) Biodiversity of freshwater fungi. J Ind Microbiol 17(5–6):328–345
Goh TK, Tsui CKM (2003) Key to common dematiaceous hyphomycetes from freshwater. In: Tsui CKM, Hyde KD (eds) Freshwater mycology. Fungal Diversity Press, Hong Kong
Goh TK, Ho WH, Hyde KD, Umali TE (1997) New records and species of Sporoschisma and Sporoschismopsis from submerged wood in the tropics. Mycol Res 101:1295–1307
Goh TK, Ho WH, Hyde KD, Whitton SR, Umali TE (1998) New records and species of Canalisporium (Hyphomycetes), with a revision of the genus. Can J Bot-Revue Canadienne De Botanique 76(1):142–152, PubMed PMID: WOS:000073021500017
Gönczöl J (1989) Longitudinal distribution patterns of aquatic hyphomycetes in a mountain stream in Hungary—experiments with leaf packs. Nova Hedwigia 48(3–4):391–404, PubMed PMID: WOS:A1989AD12000009
Gönczöl J, Révay A (2003) Treehole fungal communities: aquatic, aero-aquatic and dematiaceous hyphomycetes. Fungal Divers 12:19–34, PubMed PMID: WOS:000181503800003
Gönczöl J, Révay A (2004) Fungal spores in rainwater: stemflow, throughfall and gutter conidial assemblages. Fungal Divers 16:67–86, PubMed PMID: WOS:000222743900006
Gönczöl J, Révay A (2006) Species diversity of rainborne hyphomycete conidia from living trees. Fungal Divers 22:37–54, PubMed PMID: WOS:000239781700003
Graça MAS, Ferreira V, Canhoto C, Encalada AC, Guerrero-Bolano F, Wantzen KM et al (2015) A conceptual model of litter breakdown in low order streams. Int Rev Hydrobiol 100(1):1–12. doi:10.1002/iroh.201401757, PubMed PMID: WOS:000347777600001
Gulis V (2001) Are there any substrate preferences in aquatic hyphomycetes? Mycol Res 105:1088–1093. doi:10.1016/s0953-7562(08)61971-1, PubMed PMID: WOS:000171808900009
Gulis V, Suberkropp K (2003) Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw Biol 48(1):123–134. doi:10.1046/j.1365-2427.2003.00985.x, PubMed PMID: WOS:000179693900011
Hameed AAA, El Hawarry S, Kamel MM (2008) Prevalence and distribution of airborne and waterborne fungi and actinomycetes in the Nile river. Aerobiologia 24(4):231–240. doi:10.1007/s10453-008-9101-7, PubMed PMID: WOS:000260611900006
Harrop BL, Marks JC, Watwood ME (2009) Early bacterial and fungal colonization of leaf litter in Fossil Creek, Arizona. J N Am Benthol Soc 28(2):383–396. doi:10.1899/08-068.1, PubMed PMID: WOS:000266645700011
Heinrichs G, Hübner I, Schmidt CK, de Hoog GS, Haase G (2013) Analysis of black fungal biofilms occurring at domestic water taps. I: compositional analysis using Taq-Encoded FLX Amplicon Pyrosequencing. Mycopathologia 175:387–397
Hori C, Ishida T, Igarashi K, Samejima M, Suzuki H, Master E et al (2014) Analysis of the Phlebiopsis gigantea genome, transcriptome and secretome provides insight into its pioneer colonization strategies of wood. PLoS Genet 10, e1004759
Hu DM, Cai L, Jones EBG, Zhang H, Boonyuen N, Hyde KD (2014) Taxonomy of filamentous asexual fungi from freshwater habitats, links to sexual morphs and their phylogeny. In: Hyde KD, Jones EBG, Pang KL (eds) Freshwater fungi: and fungal-like organisms. Walter de Gruyter GmbH & Co KG, Berlin
Hyde KD, Wong SW, Jones EBG (1997) Freshwater ascomycetes. In: Hyde KD (ed) Biodiversity of tropical microfungi Hong Kong. Hong Kong University Press, Hong Kong
Ibelings BW, De Bruin A, Kagami M, Rijkeboer M, Brehm M, van Donk E (2004) Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota). J Phycol 40(3):437–453. doi:10.1111/j.1529-8817.2004.03117.x, PubMed PMID: WOS:000221644400001
Ingold CT (1942) Aquatic hyphomycetes of decaying alder leaves. Trans Br Mycol Soc 25:339–417
Ingold CT (1955) Aquatic Ascomycetes: further species from the English Lake District. Trans Br Mycol Soc 38:157–168
Ingold CT (1966) The tetraradiate aquatic fungal spore. Mycologia 58(1):43–56. doi:10.2307/3756987, PubMed PMID: WOS:A19667371900002
Ingold CT (1975) Hooker lecture 1974: convergent evolution in aquatic fungi: the tetraradiate spore. Biol J Linn Soc 7(1):1–25. doi:10.1111/j.1095-8312.1975.tb00731.x
Ingold CT, Chapman B (1952) Aquatic Ascomycetes: Loramyces juncicola Weston and L. macrospora n. sp. Trans Br Mycol Soc 35:269–272
Ishii N, Ishida S, Kagami M (2015) PCR primers for assessing community structure of aquatic fungi including Chytridiomycota and Cryptomycota. Fungal Ecol 13:33–43
James TY, Pelin A, Bonen L, Ahrendt S, Sain D, Corradi N et al (2013) Shared signatures of parasitism and phylogenomics unite Cryptomycota and Microsporidia. Curr Biol 23(16):1548–1553. doi:10.1016/j.cub.2013.06.057, PubMed PMID: WOS:000323401100019
Jones EBG (1981) Observations on the ecology of lignicolous aquatic hyphomycetes. In: Wicklow DT, Carroll GC (eds) The fungal community. Marcel Dekker, New York, pp 731–742
Jones EBG (2006) Form and function of fungal spore appendages. Mycoscience 47:167–183
Jones MDM, Forn I, Gadelha C, Egan MJ, Bass D, Massana R et al (2011) Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474(7350):200–203. doi:10.1038/nature09984, PubMed PMID: WOS:000291397800047
Jones EBG, Hyde KD, Pang KL (2014) Freshwater fungi and fungal-like organisms. Walter de Gruyter GmbH, Berlin
Junghanns C, Moeder M, Krauss G, Martin C, Schlosser D (2005) Degradation of the xenoestrogen nonylphenol by aquatic fungi and their laccases. Microbiology 151:45–57. doi:10.1099/mic.0.27431-0, PubMed PMID: WOS:000226352800006
Kagami M, Miki T, Takimoto G (2014) Mycoloop: chytrids in aquatic food webs. Front Microbiol 5:9. doi:10.3389/fmicb.2014.00166, PubMed PMID: WOS:000334664400001
Kaushik NK, Hynes HBN (1971) The fate of the dead leaves that fall into streams. Archiv Fur Hydrobiologie 68:465–515
Kelly JJ, Bansal A, Winkelman J, Janus LR, Hell S, Wencel M et al (2010) Alteration of microbial communities colonizing leaf litter in a temperate woodland stream by growth of trees under conditions of elevated atmospheric CO2. Appl Environ Microbiol 76(15):4950–4959. doi:10.1128/aem.00221-10, PubMed PMID: WOS:000280266200005
Kerr JL, Baldwin DS, Tobin MJ, Puskar L, Kappen P, Rees GN et al (2013) High spatial resolution infrared micro-spectroscopy reveals the mechanism of leaf lignin decomposition by aquatic fungi. PLoS One 8, e60857
Kohout P, Sykorova Z, Ctvrtlikova M, Rydlova J, Suda J, Vohnik M et al (2012) Surprising spectra of root-associated fungi in submerged aquatic plants. FEMS Microbiol Ecol 80(1):216–235. doi:10.1111/j.1574-6941.2011.01291.x, PubMed PMID: WOS:000301051600019
Kohout P, Tesitelova T, Roy M, Vohnik M, Jersakova J (2013) A diverse fungal community associated with Pseudorchis albida (Orchidaceae) roots. Fungal Ecol 6(1):50–64. doi:10.1016/j.funeco.2012.08.005, PubMed PMID: WOS:000314256500007
Krauss G, Bärlocher F, Schreck P, Wennrich R, Glasser W, Krauss GJ (2001) Aquatic hyphomycetes occur in hyperpolluted waters in Central Germany. Nova Hedwigia 72(3–4):419–428, PubMed PMID: WOS:000169242400010
Krauss G, Sridhar KR, Bärlocher F (2005) Aquatic hyphomycetes and leaf decomposition in contaminated groundwater wells in Central Germany. Archiv Fur Hydrobiologie 162(3):417–429. doi:10.1127/003-9136/2005/0162/0417, PubMed PMID: WOS:000228391300009
Kuske CR, Hesse CN, Challacombe JF, Cullen D, Herr JR, Mueller RC et al (2015) Prospects and challenges for fungal metatranscriptomics of complex communities. Fungal Ecol 14:133–137
Lazarus KL, James TY (2015) Surveying the biodiversity of the Cryptomycota using a targeted PCR approach. Fungal Ecol 14:62–70
Lecerf A, Richardson JS (2010a) Biodiversity-ecosystem function research: insights gained from streams. River Res Appl 26(1):45–54. doi:10.1002/rra.1286, PubMed PMID: WOS:000274310500006
Lecerf A, Richardson JS (2010b) Litter decomposition can detect effects of high and moderate levels of forest disturbance on stream condition. For Ecol Manag 259(12):2433–2443. doi:10.1016/j.foreco.2010.03.022, PubMed PMID: WOS:000278303700022
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91(2):219–227. doi:10.2307/3761366, PubMed PMID: WOS:000079317100001
Magyar D, Gönczöl J, Révay A, Grillenzoni F, Seijo-Coello MDC (2005) Stauro- and scolecoconidia in floral and honeydew honeys. Fungal Divers 20:103–120, PubMed PMID: WOS:000234463100008
Masclaux H, Perga ME, Kagami M, Desvilettes C, Bourdier G, Bec A (2013) How pollen organic matter enters freshwater food webs. Limnol Oceanogr 58:1185–1195
Miura A, Urabe J (2014) Spatial and seasonal changes in species diversity of epilithic fungi along environmental gradients of a river. Freshw Biol 60:673–685
Nagy LA, Olson BH (1982) The occurrence of filamentous fungi in drinking-water distribution-systems. Can J Microbiol 28(6):667–671, PubMed PMID: WOS:A1982NV58700017
Nechwatal J, Wielgoss A, Mendgen K (2005) Pythium phragmitis sp. nov., a new species close to P. arrhenomanes as a pathogen of common reed (Phragmites australis). Mycol Res 109:1337–1346. doi:10.1017/s0953756205003990, PubMed PMID: WOS:000233734000003
Nemec S (1969) Sporulation and identification of fungi isolated from root rot-diseased strawberry plants. Phytopathology 59(10):1552–1553, PubMed PMID: WOS:A1969E429700058
Nikolcheva LG, Bärlocher F (2005) Seasonal and substrate preferences of fungi colonizing leaves in streams: traditional versus molecular evidence. Environ Microbiol 7(2):270–280. doi:10.1111/j.1462-2920.2004.00709.x, PubMed PMID: WOS:000226376800013
Nikolcheva LG, Cockshutt AM, Bärlocher F (2003) Determining diversity of freshwater fungi on decaying leaves: comparison of traditional and molecular approaches. Appl Environ Microbiol 69(5):2548–2554. doi:10.1128/aem/69.5.2548.2554.2003, PubMed PMID: WOS:000182808300015
Nikolcheva LG, Bourque T, Bärlocher F (2005) Fungal diversity during initial stages of leaf decomposition in a stream. Mycol Res 109:246–253. doi:10.1017/s0953756204001698
Niyogi DK, Cheatham CA, Thomson WH, Christiansen JM (2009) Litter breakdown and fungal diversity in a stream affected by mine drainage. Fundam Appl Limnol 175(1):39–48. doi:10.1127/1863-9135/2009/0175-0039, PubMed PMID: WOS:000269326500003
Noga EJ (1993) Fungal diseases of marine and estuarine fishes. In: Couch JA, Fournie JW (eds) Pathology of marine and estuarine organisms. CRC Press, Boca Raton, FL, pp 85–110
Park D (1974) Aquatic hyphomycetes in non-aquatic habitats. Trans Br Mycol Soc 63(1):183–187
Pascoal C, Cassio F, Marvanová L (2005) Anthropogenic stress may affect aquatic hyphomycete diversity more than leaf decomposition in a low-order stream. Archiv Fur Hydrobiologie 162(4):481–496. doi:10.1127/0003-9136/2005/0162-0481, PubMed PMID: WOS:000229072700004
Pérez J, Descals E, Pozo J (2012) Aquatic Hyphomycete communities associated with decomposing alder leaf litter in reference headwater streams of the Basque Country (northern Spain). Microb Ecol 64(2):279–290. doi:10.1007/s00248-012-0022-1, PubMed PMID: WOS:000306174700001
Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 57:20–33
Powell MJ (1993) Looking at mycology with a Janus face: a glimpse at Chytridiomycetes active in the environment. Mycologia 85:1–20
Powell MJ, Letcher PM (2014) 6 Chytridiomycota, Monoblepharidomycota, and Neocallimastigomycota. In: McLaughlin DJ, Spatafora JW (eds) Systematics and evolution. 7A, 2nd edn. Springer, Berlin, pp 141–175
Powell MJ, Letcher PM, Blackwell WH (2013) A new aquatic cellulose-degrading chytrid in the Chytridiales. Phytopathology 103(6):115, PubMed PMID: WOS:000322799500634
Raja HA, Schmit JP, Shearer CA (2009) Latitudinal, habitat and substrate distribution patterns of freshwater ascomycetes in the Florida Peninsula. Biodivers Conserv 18(2):419–455. doi:10.1007/s10531-008-9500-7, PubMed PMID: WOS:000262965400011
Raja HA, Oberlies NH, Figueroa M, Tanaka K, Hirayama K, Hashimoto A et al (2013) Freshwater Ascomycetes: Minutisphaera (Dothideomycetes) revisited, including one new species from Japan. Mycologia 105(4):959–976. doi:10.3852/12-313, PubMed PMID: WOS:000322849500014
Raviraja NS, Sridhar KR, Bärlocher F (1998) Breakdown of Ficus and Eucalyptus leaves in an organically polluted river in India: fungal diversity and ecological functions. Freshw Biol 39:537–545
Reynolds JD (1988) Crayfish extinctions and crayfish plague in central Ireland. Biol Conserv 45(4):279–285. doi:10.1016/0006-3207(88)90059-6, PubMed PMID: WOS:A1988P839500004
Sati SC, Belwal M (2005) Aquatic hyphomycetes as endophytes of riparian plant roots. Mycologia 97(1):45–49. doi:10.3852/mycologia.97.1.45, PubMed PMID: WOS:000229365800005
Schlütz F, Shumilovskikh LS (2013) On the relation of Potamomyces armatisporus to the fossil form-type Mediaverrunites and its taxonomical and ecological implications. Fungal Ecol 6(4):309–315
Schulz B, Boyle C (2005) The endophytic continuum. Mycol Res 109:661–686. doi:10.1017/s095375620500273x, PubMed PMID: WOS:000230687600001
Seena S, Wynberg N, Baerlocher F (2008) Fungal diversity during leaf decomposition in a stream assessed through clone libraries. Fungal Divers 30:1–14, PubMed PMID: WOS:000258548800001
Selosse MA, Vohnik M, Chauvet E (2008) Out of the rivers: are some aquatic hyphomycetes plant endophytes? New Phytol 178(1):3–7. doi:10.1111/j.1469-8137.2008.02390.x, PubMed PMID: WOS:000253711800002
Shearer CA (1993a) The fresh-water Ascomycetes. Nova Hedwigia 56(1–2):1–33, PubMed PMID: WOS:A1993KT94300001
Shearer CA (1993b) A new species of Kirschsteiniothelia (Pleosporales) with an unusual fissitunicate ascus. Mycologia 85:963–969
Shearer CA, Webster J (1985a) Aquatic hyphomycete communities in the River Teign. 1. Longitudinal distribution patterns. Trans Br Mycol Soc 84:489–501, PubMed PMID: WOS:A1985AHE9100011
Shearer CA, Webster J (1985b) Aquatic hyphomycete communities in the River Teign. 3. Comparison of sampling techniques. Trans Br Mycol Soc 84:509–518, PubMed PMID: WOS:A1985AHE9100013
Shearer CA, Webster J (1991) Aquatic hyphomycete communities in the River Teign. 4. Twig colonization. Mycol Res 95:413–420, PubMed PMID: WOS:A1991FL70400006
Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanová L, Padgett D et al (2007) Fungal biodiversity in aquatic habitats. Biodivers Conserv 16(1):49–67. doi:10.1007/s10531-006-9120-z, PubMed PMID: WOS:000244185900004
Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, Hirayama K et al (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153. doi:10.3114/sim.2009.64.08, PubMed PMID: WOS:000274365900009
Simonis JL, Raja HA, Shearer CA (2008) Extracellular enzymes and soft rot decay: are ascomycetes important degraders in freshwater? Fungal Divers 31:135–146
Sing VO, Bartnicki-Garcia S (1972) Adhesion of zoospores of Phytophthora palmivora to solid surface. Phytopathology 62:790
Singh S, Harms H, Schlosser D (2014) Screening of ecologically diverse fungi for their potential to pretreat lignocellulosic bioenergy feedstock. Appl Microbiol Biotechnol 98(7):3355–3370. doi:10.1007/s00253-014-5563-4, PubMed PMID: WOS:000334167900045
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD et al (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4(2):125–134. doi:10.1007/s10393-007-0093-5, PubMed PMID: WOS:000248231900004
Smither-Kopperl ML, Charudattan R, Berger RD (1998) Dispersal of spores of Fusarium culmorum in aquatic systems. Phytopathology 88(5):382–388. doi:10.1094/phyto.1998.88.5.382, PubMed PMID: WOS:000073487300002
Sole M, Muller I, Pecyna MJ, Fetzer I, Harms H, Schlosser D (2012) Differential regulation by organic compounds and heavy metals of multiple laccase genes in the aquatic Hyphomycete Clavariopsis aquatica. Appl Environ Microbiol 78(13):4732–4739. doi:10.1128/aem.00635-12, PubMed PMID: WOS:000305376600023
Sondergaard M, Laegaard S (1977) Vesicular-arbuscular mycorrhiza in some aquatic vascular plants. Nature 268(5617):232–233. doi:10.1038/268232a0, PubMed PMID: WOS:A1977DN97200031
Sonstebo JH, Rohrlack T (2011) Possible implications of chytrid parasitism for population subdivision in freshwater cyanobacteria of the genus Planktothrix. Appl Environ Microbiol 77(4):1344–1351. doi:10.1128/aem.02153-10, PubMed PMID: WOS:000287078100023
Sparrow FK (1960) Aquatic phycomycetes. University of Michigan Press, Ann Arbor, MI, 1187 p
Sridhar KR, Bärlocher F, Krauss GJ, Krauss G (2005) Response of aquatic hyphomycete communities to changes in heavy metal exposure. Int Rev Hydrobiol 90(1):21–32. doi:10.1002/iroh.200410739, PubMed PMID: WOS:000227722800002
Sridhar KR, Duarte S, Cassio F, Pascoal C (2009) The role of early fungal colonizers in leaf-litter decomposition in Portuguese streams impacted by agricultural runoff. Int Rev Hydrobiol 94(4):399–409. doi:10.1002/iroh.200811154, PubMed PMID: WOS:000269832100005
Stajich JEBM, Blackwell M, Hibbett DS, James TY, Spatafora JW, Taylor JW (2009) The fungi. Curr Biol 19:R840–R845
Sudova R, Rydlova J, Ctvrtlikova M, Havranek P, Adamec L (2011) The incidence of arbuscular mycorrhiza in two submerged Isoetes species. Aquat Bot 94(4):183–187. doi:10.1016/j.aquabot.2011.02.003, PubMed PMID: WOS:000290502100006
Taylor JW, Berbee ML (2006) Dating divergences in the fungal tree of life: review and new analyses. Mycologia 98:838–849
Tedersoo L, Pellet P, Kõljalg U, Selosse M-A (2007) Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151(2):206–217
Thomas K, Chilvers GA, Norris RH (1989) Seasonal occurrence of conidia of aquatic hyphomycetes (fungi) in Lees Creek, Australian Capital Territory. Aust J Mar Freshwat Res 40:11–23
Thomas K, Chilvers GA, Norris RH (1992) Aquatic hyphomycetes from different substrates; substrate preference and seasonal occurrence. Aust J Mar Freshwat Res 43:491–509
Tsui CKM, Berbee ML (2006) Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Mol Phylogenet Evol 39(3):587–597. doi:10.1016/j.ympev.2006.01.025, PubMed PMID: WOS:000238155300001
Tsui CKM, Hyde KD, Hodgkiss IJ (2001a) Longitudinal and temporal distribution of freshwater ascomycetes and dematiaceous hyphomycetes on submerged wood in the Lam Tsuen River, Hong Kong. J N Am Benthol Soc 20(4):533–549. doi:10.2307/1468086, PubMed PMID: WOS:000172517400003
Tsui CKM, Hyde KD, Hodgkiss IJ (2001b) Colonization patterns of wood-inhabiting fungi on baits in Hong Kong rivers, with reference to the effects of organic pollution. Anton Leeuw Int J Gen Mol Microbiol 79(1):33–38. doi:10.1023/a:1010210631215, PubMed PMID: WOS:000167920200004
Tubaki K, Tokumasu S, Ando K (1985) Morning dew and Tripospermum (Hyphomycetes). Bot J Linn Soc 91:45–50
Vijaykrishna D, Jeewon R, Hyde KD (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers 23:351–390, PubMed PMID: WOS:000243414100015
Voglmayr H (2004) Spirosphaera cupreorufescens sp. nov., a rare aeroaquatic fungus. Stud Mycol 50(1):221–228
Voglmayr H (2011) Phylogenetic relationships and reclassification of Spirosphaera lignicola, an enigmatic aeroaquatic fungus. Mycotaxon 116:191–202
Voglmayr H, Delgado-Rodriguez G (2001) Dendroclathra caeruleofusca gen.nov. et sp.nov., an aeroaquatic hyphomycete from Cuba. Can J Bot-Revue Canadienne De Botanique 79(9):995–1000, PubMed PMID: WOS:000171070800001
Voglmayr H, Delgado-Rodriguez G (2003) New species, notes and key to the aeroaquatic genera Beverwykella and Ramicephala gen. nov. Mycol Res 107:236–244. doi:10.1017/s0953756203007172, PubMed PMID: WOS:000182647300015
Voglmayr H, Krisai-Greilhuber I (1997) Pseudoclathrosphaerina evamariae gen. et sp. nov. and Sympodioclathra globosa gen. et sp. nov., two aeroaquatic fungi similar to Clathrosphaerina. Mycologia 89(6):942–951. doi:10.2307/3761115, PubMed PMID: WOS:A1997YK93200014
Voglmayr H, Park MJ, Shin HD (2011) Spiroplana centripeta gen. & sp. nov., a leaf parasite of Philadelphus and Deutzia with a remarkable aeroaquatic conidium morphology. Mycotaxon 116:203–216
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Watanabe T (1975) Tetracladium setigerum an aquatic hyphomycetes associated with gentian and strawberry roots. Trans Mycol Soc Jpn 16:348–350
Webster J (1959) Experiments with spores of aquatic hyphomycetes I: sedimentation and impaction on smooth surfaces. Ann Bot 23:595–611
Webster J (1980) Introduction to fungi, 2nd edn. Cambridge University Press, Cambridge
Webster J, Davey RA (1984) Sigmoid conidial shape in aquatic fungi. Trans Br Mycol Soc 83:43–52, PubMed PMID: WOS:A1984TH62300005
Webster J, Descals E (1981) Morphology, distribution, and ecology of conidial fungi in freshwater habitats. In: Cole GC, Kendrick B (eds) Biology of conidial fungi. Academic, London, pp 295–335
Willoughby LG (2003) Diseases of freshwater fishes. In: Tsui CKM, Hyde KD (eds) Freshwater mycology. Fungal Diversity Press, Hong Kong, pp 111–126
Wong SW, Hyde KD (1999) Proboscispora aquatica gen. et sp. nov., from wood submerged in freshwater. Mycol Res 103:81–87. doi:10.1017/s0953756298006820, PubMed PMID: WOS:000078869900013
Wong MKM, Goh TK, Hodgkiss IJ, Hyde KD, Ranghoo VM, Tsui CKM et al (1998) Role of fungi in freshwater ecosystems. Biodivers Conserv 7(9):1187–1206. doi:10.1023/a:1008883716975, PubMed PMID: WOS:000078129900005
Wood-Eggenschwiler S, Bärlocher F (1985) Geographical distribution of Ingoldian fungi. Verhandlungen Internationaler nereinigung fuer Theoretische und Angewanot d Liminologie 22:2780–2785
Wurzbacher C, Rosel S, Rychla A, Grossart H-P (2014) Importance of saprotrophic freshwater fungi for pollen degradation. PLoS One 9(4), e94643
Yuen TK, Hyde KD, Hodgkiss IJ (1998) Physiological growth parameters and enzyme production in tropical freshwater fungi. Mater Org 32(1):1–16, PubMed PMID: WOS:000079120700001
Zemek J, Marvanov L, Kuniak L, Kadlecikova B (1985) Hydrolytic enzymes in aquatic hyphomycetes. Folia Microbiol 30:363–372
Acknowledgments
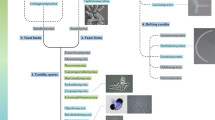
The authors wish to thank the following students of Professor T.K. Goh, for providing the pictures of freshwater fungi used in this chapter: Bao Yee Loh (Oomycetes), Huey Kei Lee (Ingoldian fungi), and Wee Kee Sim (lignicolous hyphomycetes). We are grateful to Dr. Huzefa Raja for comments and suggestions. The authors thank the master student of Christiane Baschien, Jens Schmidt for the picture of Lunulospora curvula conidiogenesis.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Our work is dedicated to John Webster (1925–2014) for his pioneer and influential work on freshwater fungi.
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Tsui, C.K.M., Baschien, C., Goh, TK. (2016). Biology and Ecology of Freshwater Fungi. In: Li, DW. (eds) Biology of Microfungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-29137-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-29137-6_13
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29135-2
Online ISBN: 978-3-319-29137-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)