Abstract
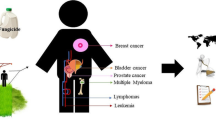
Pesticides are widely used in agriculture to control weeds, insects, fungi, rodents, and other pests, but their use can have negative effects on both the environment and human health. This article discusses the various ways in which individuals can be exposed to pesticides and the potential health effects of such exposure. Growing evidence suggests that exposure to pesticides can increase the risk of developing cancer, with any class of pesticides being a risk factor. Specific chemicals in many classes of pesticides have been linked to various types of cancer. This article discusses the link between pesticide exposure and various types of cancer, including lung, breast, genitourinary, leukemia, brain, and myeloma. Efforts should be made to limit exposure to reduce cancer incidence, particularly with regard to certain pesticides such as organochlorines, triazines, organophosphates, chlorophenoxy, pyrethroids, and carbamates. While some pesticides are not considered carcinogenic due to a lack of evidence, in vitro and in vivo studies have shown characteristics of carcinogens for some pesticides. Further studies are required to evaluate the carcinogenicity of pesticides and reduce disparities in cancer risks.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Atwood D, Paisley-Jones C. Pesticides industry sales and usage: 2008–2012 market estimates. US Environmental Protection Agency; 2017.
Tudi M, Li H, Li H, et al. Exposure routes and health risks associated with pesticide application. Toxics. 2022;10(6):335.
Gangemi S, Miozzi E, Teodoro M, et al. Occupational exposure to pesticides as a possible risk factor for the development of chronic diseases in humans. Mol Med Rep. 2016;14(5):4475–88.
Wang Y, Abd El-Aty AM, Wang S, et al. Competitive fluorescent immunosensor based on catalytic hairpin self-assembly for multiresidue detection of organophosphate pesticides in agricultural products. Food Chem. 2023;413:5607.
De Troeyer K, Casas L, Bijnens EM, et al. Higher proportion of agricultural land use around the residence is associated with higher urinary concentrations of AMPA, a glyphosate metabolite. Int J Hyg Environ Health. 2022;246:114039.
Gunier RB, Ward MH, Airola M, et al. Determinants of agricultural pesticide concentrations in carpet dust. Environ Health Perspect. 2011;119(7):970–6.
Harnly ME, Bradman A, Nishioka M, et al. Pesticides in dust from homes in an agricultural area. Environ Sci Technol. 2009;43(23):8767–74.
Bradman A, Castorina R, Boyd Barr D, et al. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int J Environ Res Public Health. 2011;8(4):1061–83.
Cecchi A, Alvarez G, Quidel N, et al. Residential proximity to pesticide applications in Argentine Patagonia: impact on pregnancy and newborn parameters. Environ Sci Pollut Res. 2021;28(40):56565–79.
US Department of Agriculture. 2022. Pesticide Data Program Annual Summary Calendar Year 2021.
Bexfield LM, Belitz K, Lindsey BD, et al. Pesticides and pesticide degradates in groundwater used for public supply across the United States: occurrence and human-health context. Environ Sci Technol. 2020;55(1):362–72.
Desimone L, Hamilton PA, Gilliom RJ. 2009. Quality of water from domestic wells in principal aquifers of the United States, 1991–2004. National Water Quality Assessment Program, Circular 1332. US Geological Survey. https://pubs.usgs.gov/circ/circ1332/includes/circ1332.pdf. Accessed 13 Dec 2022.
Levy ZF, Balkan M, Shelton JL. 2023. Quality of groundwater used for domestic supply in the Modesto, Turlock, and Merced Subbasins of the San Joaquin Valley, California. US Geological Survey. https://pubs.usgs.gov/of/2022/1116/ofr20221116.pdf. Accessed 11 Feb 2023.
California Environmental Health Protection Agency. 2023. About Proposition 65. https://oehha.ca.gov/proposition-65/about-proposition-65. Accessed 07 Feb 2023.
International Agency for Research on Cancer. 2019. IARC Monographs on the identification of carcinogenic hazards to humans – preamble. https://monographs.iarc.who.int/wp-content/uploads/2019/07/Preamble-2019.pdf. Accessed 07 Feb 2023.
US Environmental Protection Agency. 2022. Evaluating pesticides for carcinogenic potential. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/evaluating-pesticides-carcinogenic-potential. Accessed 08 Feb 2023.
International Agency for Research on Cancer. 2023. Agents classified by the IARC monographs, vols 1–129. http://monographs.iarc.fr/ENG/Classification/index.php. Accessed 07 Feb 2023.
US Environmental Protection Agency. 2022. Chemicals evaluated for carcinogenic potential.
Sharma R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol. 2022;27(4):665–75.
National Cancer Institute. 2023. Cancer stat facts: lung and bronchus cancer, surveillance, epidemiology, and end results program. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 08 Feb 2023.
Szalontai K, Gémes N, Furák J, et al. Chronic obstructive pulmonary disease: epidemiology, biomarkers, and paving the way to lung cancer. J Clin Med. 2021;10(13):2889.
Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res Genet Toxicol Environ Mutagen. 2006;608(2):157–62.
Alavanja MC, Bonner MR. Occupational pesticide exposures and cancer risk: a review. J Toxicol Environ Health B Crit Rev. 2012;15(4):238–63.
Zendehdel R, Tayefeh-Rahimian R, Kabir A. Chronic exposure to chlorophenol related compounds in the pesticide production workplace and lung cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(13):5149–53.
Boulanger M, Tual S, Lemarchand C, et al. Lung cancer risk and occupational exposures in crop farming: results from the AGRIculture and CANcer (AGRICAN) cohort. Occup Environ Med. 2018;75(11):776–85.
Jones RR, Barone-Adesi F, Koutros S, et al. Incidence of solid tumours among pesticide applicators exposed to the organophosphate insecticide diazinon in the Agricultural Health Study: an updated analysis. Occup Environ Med. 2015;72(7):496–503.
Lerro CC, Koutros S, Andreotti G, et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med. 2015a;72(10):736–44.
Lerro CC, Koutros S, Andreotti G, et al. Use of acetochlor and cancer incidence in the Agricultural Health Study. Int J Cancer. 2015b;137(5):1167–75.
Bonner MR, Freeman LE, Hoppin JA, et al. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ Health Perspect. 2017;125(4):544–51.
Kim B, Park EY, Kim J, et al. Occupational exposure to pesticides and lung cancer risk: a propensity score analyses. Cancer Res Treat. 2022;54(1):130–9.
Parron T, Requena M, Hernandez AF, et al. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol Lett. 2014;230(2):157–65.
Liu CT, Yang CC, Chien WC, et al. Association between long-term usage of acetylcholinesterase inhibitors and lung cancer in the elderly: a nationwide cohort study. Sci Rep. 2022;12(1):3531.
Majidi M, Delirrad M, Banagozar Mohammadi A, et al. Cholinesterase level in erythrocyte or serum: which is more predictive of the clinical outcome in patients with acute organophosphate poisoning? Iran J Toxicol. 2018;12(5):23–6.
Xi HJ, Wu RP, Liu JJ, et al. Role of acetylcholinesterase in lung cancer. Thorac Cancer. 2015;6(4):390–8.
Ziech D, Franco R, Georgakilas AG, et al. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact. 2010;188(2):334–9.
Thakur S, Dhiman M, Mantha AK. APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Mol Cell Biochem. 2018;441:201–16.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Høyer AP, Jørgensen T, Rank F, et al. Organochlorine exposures influence on breast cancer risk and survival according to estrogen receptor status: a Danish cohort-nested case-control study. BMC Cancer. 2001;1(1):1–8.
Ferro R, Parvathaneni A, Patel S, et al. Pesticides and breast cancer. Adv Breast Cancer Res. 2012;1(3):30–5.
Cabello G, Valenzuela-Estrada M, Siques P, et al. Relation of breast cancer and malathion aerial spraying in Arica, Chile. Int J Morphol. 2013;31(2):640–5.
Arrebola JP, Belhassen H, Artacho-Cordón F, et al. Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: a case–control study in Tunisia. Sci Total Environ. 2015;520:106–13.
Cohn BA, Cirillo PM, Terry MB. DDT and breast cancer: prospective study of induction time and susceptibility windows. J Natl Cancer Inst. 2019;111(8):803–10.
Tayour C, Ritz B, Langholz B, et al. A case–control study of breast cancer risk and ambient exposure to pesticides. Environ Epidemiol. 2019;3(5)
Mekonen S, Ibrahim M, Astatkie H, et al. Exposure to organochlorine pesticides as a predictor to breast cancer: A case-control study among Ethiopian women. PLoS One. 2021;16(9):e0257704.
Franke AA, Li X, Shvetsov YB, et al. Pilot study on the urinary excretion of the glyphosate metabolite aminomethylphosphonic acid and breast cancer risk: the Multiethnic Cohort study. Environ Pollut. 2021;277:116848.
Eldakroory SA, Morsi DE, Abdel-Rahman RH, et al. Correlation between toxic organochlorine pesticides and breast cancer. Hum Exp Toxicol. 2017;36(12):1326–34.
Kaur N, Swain SK, Banerjee BD, et al. Organochlorine pesticide exposure as a risk factor for breast cancer in young Indian women: a case–control study. South Asian J Cancer. 2019;8(4):212–4.
Wallace DR. Environmental pesticides and heavy metals - role in breast cancer. In: Larramendy ML, Solonesk S, editors. Toxicity and hazard of agrochemicals. London: IntechOpen; 2015. p. p39–70.
Yang KJ, Lee J, Park HL. Organophosphate pesticide exposure and breast cancer risk: a rapid review of human, animal, and cell-based studies. Int J Environ Res Public Health. 2020;17(14):5030.
Pizzatti L, Kawassaki ACB, Fadel B, et al. Toxicoproteomics disclose pesticides as downregulators of TNF-α, IL-1β and estrogen receptor pathways in breast cancer women chronically exposed. Front Oncol. 2020;10:1698.
Cardona B, Rudel RA. US EPA’s regulatory pesticide evaluations need clearer guidelines for considering mammary gland tumors and other mammary gland effects. Mol Cell Endocrinol. 2020;518:110927.
Green BL, Davis JL, Rivers D, et al. Cancer health disparities. In: Alberts DS, Hess LM, editors. Fundamentals of cancer prevention. 4th ed. Cham: Springer; 2019. p. p199–246.
Landau-Ossondo M, Rabia N, Jos-Pelage J, et al. Why pesticides could be a common cause of prostate and breast cancers in the French Caribbean Island, Martinique. An overview on key mechanisms of pesticide-induced cancer. Biomed Pharmacother. 2009;63(6):383–95.
Settimi L, Masina A, Andrion A, et al. Prostate cancer and exposure to pesticides in agricultural settings. Int J Cancer. 2003;104(4):458–61.
Multigner L, Ndong JR, Giusti A, et al. Chlordecone exposure and risk of prostate cancer. J Clin Oncol. 2010;28(21):3457–62.
Band PR, Abanto Z, Bert J, et al. Prostate cancer risk and exposure to pesticides in British Columbia farmers. Prostate. 2011;71(2):168–83.
Abhishek A, Ansari NG, Singh V, et al. Genetic susceptibility of CYP1A1 gene and risk of pesticide exposure in prostate cancer. Cancer Biomark. 2020;29(4):429–40.
Koutros S, Beane Freeman LE, Lubin JH, et al. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol. 2013;177(1):59–74.
Pardo LA, Beane Freeman LE, Lerro CC, et al. Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ Health. 2020;19(1):1–12.
Cockburn M, Mills P, Zhang X, et al. Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. Am J Epidemiol. 2011;173(11):1280–8.
Lewis-Mikhael AM, Bueno-Cavanillas A, Guiron TO, et al. Occupational exposure to pesticides and prostate cancer: a systematic review and meta-analysis. Occup Environ Med. 2016;73(2):134–44.
Silva JF, Mattos IE, Luz LL, et al. Exposure to pesticides and prostate cancer: systematic review of the literature. Rev Environ Health. 2016;31(3):311–27.
Djalali-Behzad G, Hussain S, Osterman-Golkar S, et al. Estimation of genetic risks of alkylating agents: VI. Exposure of mice and bacteria to methyl bromide. Mutat Res. 1981;84(1):1–9.
Pletsa V, Steenwinkel MJ, van Delft JH, et al. Methyl bromide causes DNA methylation in rats and mice but fails to induce somatic mutations in λlacZ transgenic mice. Cancer Lett. 1998;135(1):21–7.
Budnik LT, Kloth S, Velasco-Garrido M, et al. Prostate cancer and toxicity from critical use exemptions of methyl bromide: environmental protection helps protect against human health risks. Environ Health. 2012;11(5):1–13.
Tessier DM, Matsumura F. Increased ErbB-2 tyrosine kinase activity, MAPK phosphorylation, and cell proliferation in the prostate cancer cell line LNCaP following treatment by select pesticides. Toxicol Sci. 2001;60(1):38–43.
Roberto M, Panebianco M, Aschelter AM, et al. The value of the multidisciplinary team in metastatic renal cell carcinoma: paving the way for precision medicine in toxicities management. Front Oncol. 2023;12:1026978.
Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–57.
Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79–87.
Andreotti G, Beane Freeman LE, Shearer JJ, et al. Occupational pesticide use and risk of renal cell carcinoma in the agricultural health study. Environ Health Perspect. 2020;128(6):067011.
Hu J, Mao Y, White K, et al. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond). 2002;52(3):157–64.
Rios P, Bauer H, Schleiermacher G, et al. Environmental exposures related to parental habits in the perinatal period and the risk of Wilms' tumor in children. Cancer Epidemiol. 2020;66:101706.
Liu W, Du Y, Liu J, et al. Effects of atrazine on the oxidative damage of kidney in Wister rats. Int J Clin Exp Med. 2014;7(10):3235.
Sánchez OF, Lin L, Bryan CJ, et al. Profiling epigenetic changes in human cell line induced by atrazine exposure. Environ Pollut. 2020;258:113712.
Abid A, Ajaz S, Khan AR, Zehra F, et al. Analysis of the glutathione S-transferase genes polymorphisms in the risk and prognosis of renal cell carcinomas. Case-control and meta-analysis. Urol Oncol. 2016;34(9):419.e1–2.
Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of bladder cancer. Med Sci. 2020;8(1):15.
Lin W, Pan X, Zhang C, et al. Impact of age at diagnosis of bladder cancer on survival: a surveillance, epidemiology, and end results-based study 2004–2015. Cancer Control. 2023;30:10732748231152322.
Kiriluk KJ, Prasad SM, Patel AR, et al. Bladder cancer risk from occupational and environmental exposures. Urol Oncol. 2012;30(2):199–211.
Letašiová S, Medveďová A, Šovčíková A, et al. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11(1):1–5.
Sharma T, Jain S, Verma A, et al. Gene environment interaction in urinary bladder cancer with special reference to organochlorine pesticide: a case control study. Cancer Biomark. 2013;13(4):243–51.
Boada LD, Henríquez-Hernández LA, Zumbado M, et al. Organochlorine pesticides exposure and bladder cancer: evaluation from a gene-environment perspective in a hospital-based case-control study in the Canary Islands (Spain). J Agromedicine. 2016;21(1):34–42.
Koutros S, Silverman DT, Alavanja MC, et al. Occupational exposure to pesticides and bladder cancer risk. Int J Epidemiol. 2016;45(3):792–805.
Liang YJ, Long DX, Xu MY, et al. Body fluids from the rat exposed to chlorpyrifos induce cytotoxicity against the corresponding tissue− derived cells in vitro. BMC Pharmacol Toxicol. 2021;22:1–8.
Matic MG, Coric VM, Savic-Radojevic AR, et al. Does occupational exposure to solvents and pesticides in association with glutathione S-transferase A1, M1, P1, and T1 polymorphisms increase the risk of bladder cancer? The Belgrade case-control study. PLoS One. 2014;9(6):e99448.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Pourshams A, Sepanlou SG, Ikuta KS, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):934–47.
Brugel M, Carlier C, Reyes-Castellanos G, et al. Pesticides and pancreatic adenocarcinoma: a transversal epidemiological, environmental and mechanistic narrative review. Dig Liver Dis. 2022;54(12):1605–13.
Weinstein B, da Silva A, Carpenter DO. Exocrine pancreatic cancer and living near to waste sites containing hazardous organic chemicals, New York State, USA–an 18-year population-based study. Int J Occup Med Environ Health. 2022;35(4):459–71.
Porta M, Gasull M, Pumarega J, et al. Plasma concentrations of persistent organic pollutants and pancreatic cancer risk. Int J Epidemiol. 2022;51(2):479–90.
Clary T, Ritz B. Pancreatic cancer mortality and organochlorine pesticide exposure in California, 1989–1996. Am J Ind Med. 2003;43(3):306–13.
McGwin G Jr, Griffin RL. An ecologic study of the association between 1, 3-dichloropropene and pancreatic cancer. Cancers. 2022;15(1):150.
Fritschi L, Benke G, Risch HA, et al. Occupational exposure to N-nitrosamines and pesticides and risk of pancreatic cancer. Occup Environ Med. 2015;72(9):678–83.
He B, Ni Y, Jin Y, et al. Pesticides-induced energy metabolic disorders. Sci Total Environ. 2020;729:139033.
Liou GY, Döppler H, DelGiorno KE, et al. Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. 2016;14(10):2325–36.
Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of non-Hodgkin’s lymphoma. Med Sci. 2021;9(1):5.
Singh R, Shaik S, Negi BS, et al. Non-Hodgkin's lymphoma: a review. J Family Med Prim Care. 2020;9(4):1834.
Kim CJ, Freedman DM, Curtis RE, et al. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leuk Lymphoma. 2013;54(8):1691–7.
Sapkota S, Shaikh H. Non-Hodgkin lymphoma. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK559328/.
Cantor KP, Blair A, Everett G, et al. Pesticides and other agricultural risk factors for non-Hodgkin’s lymphoma among men in Iowa and Minnesota. Cancer Res. 1992;52(9):2447–55.
Meinert R, Schüz J, Kaletsch U, et al. Leukemia and non-Hodgkin's lymphoma in childhood and exposure to pesticides: results of a register-based case-control study in Germany. Am J Epidemiol. 2000;151(7):639–46.
Fritschi L, Benke G, Hughes AM, et al. Occupational exposure to pesticides and risk of non-Hodgkin's lymphoma. Am J Epidemiol. 2005;162(9):849–57.
McDuffie HH, Pahwa P, McLaughlin JR, et al. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Biomark Prev. 2001;10(11):1155–63.
Yildirim M, Karakilinc H, Yildiz M, et al. Non-Hodgkin lymphoma and pesticide exposure in Turkey. Asian Pac J Cancer Prev. 2013;14(6):3461–3.
Schinasi L, Leon ME. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(4):4449–527.
Poh C, McPherson JD, Tuscano J, et al. Environmental pesticide exposure and non-Hodgkin lymphoma survival: a population-based study. BMC Med. 2022;20(1):165.
Faivdullah L, Azahar F, Htike ZZ, et al. Leukemia detection from blood smears. J Med Biol Eng. 2015;4(6):488–91.
Sielken RL, Valdez-Flores C. A comprehensive review of occupational and general population cancer risk: 1, 3-Butadiene exposure–response modeling for all leukemia, acute myelogenous leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, myeloid neoplasm and lymphoid neoplasm. Chem Biol Interact. 2015;241:50–8.
Sakamoto KM, Grant S, Saleiro D, et al. Targeting novel signaling pathways for resistant acute myeloid leukemia. Mol Genet Metab. 2015;114(3):397–402.
Miranda-Filho AL, Piñeros M, Ferlay J, et al. Epidemiological patterns of leukaemia in 184 countries: a population-based study. Lancet Haematol. 2018;5(1):e14–24.
Shallis RM, Weiss JJ, Deziel NC, et al. Challenging the concept of de novo acute myeloid leukemia: Environmental and occupational leukemogens hiding in our midst. Blood Rev. 2021;47:100760.
Foucault A, Vallet N, Ravalet N, et al. Occupational pesticide exposure increases risk of acute myeloid leukemia: a meta-analysis of case–control studies including 3,955 cases and 9,948 controls. Sci Rep. 2021;11(1):1–13.
Brown LM, Blair A, Gibson R, et al. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 1990;50(20):6585–91.
Nguyen A, Crespi CM, Vergara X, et al. Residential proximity to plant nurseries and risk of childhood leukemia. Environ Res. 2021;200:111388.
Patel DM, Gyldenkærne S, Jones RR, et al. Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environ Int. 2020;143:105955.
Park AS, Ritz B, Yu F, et al. Prenatal pesticide exposure and childhood leukemia–a California statewide case-control study. Int J Hyg Environ Health. 2020;226:113486.
Karalexi MA, Tagkas CF, Markozannes G, et al. Exposure to pesticides and childhood leukemia risk: a systematic review and meta-analysis. Environ Pollut. 2021;285:117376.
Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45.
Farmanfarma KK, Mohammadian M, Shahabinia Z, et al. Brain cancer in the world: an epidemiological review. World Cancer Res J. 2019;6(5)
Johnson KJ, Cullen J, Barnholtz-Sloan JS, et al. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiol Biomark Prev. 2014;23(12):2716–36.
Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(S7):1953–68.
Miranda-Filho AL, Koifman RJ, Koifman S, et al. Brain cancer mortality in an agricultural and a metropolitan region of Rio de Janeiro, Brazil: a population-based, age-period-cohort study, 1996–2010. BMC Cancer. 2014;14(1):1–9.
Carreón T, Butler MA, Ruder AM, et al. Gliomas and farm pesticide exposure in women: the Upper Midwest Health Study. Environ Health Perspect. 2005;113(5):546–51.
Lee WJ, Colt JS, Heineman EF, et al. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup Environ Med. 2005;62(11):786–92.
Provost D, Cantagrel A, Lebailly P, et al. Brain tumours and exposure to pesticides: a case–control study in southwestern France. Occup Environ Med. 2007;64(8):509–14.
Bhat AR, Wani MA, Kirmani AR, et al. Pesticides and brain cancer linked in orchard farmers of Kashmir. Indian J Med Paediatr Oncol. 2010;31(4):110–20.
Shim YK, Mlynarek SP, van Wijngaarden E. Parental exposure to pesticides and childhood brain cancer: US Atlantic coast childhood brain cancer study. Environ Health Perspect. 2009;117(6):1002–6.
Zhang L, Tai YT, Ho M, et al. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017;7(3):e547.
Huang J, Chan SC, Lok V, et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022;9(9):670–7.
Viel JF, Richardson ST. Lymphoma, multiple myeloma and leukaemia among French farmers in relation to pesticide exposure. Soc Sci Med. 1993;37(6):771–7.
Brown LM, Burmeister LF, Everett GD, et al. Pesticide exposures and multiple myeloma in Iowa men. Cancer Causes Control. 1993;4:153–6.
Orsi L, Delabre L, Monnereau A, et al. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. Occup Environ Med. 2009;66(5):291–8.
Kachuri L, Demers PA, Blair A, et al. Multiple pesticide exposures and the risk of multiple myeloma in Canadian men. Int J Cancer. 2013;133(8):1846–58.
Pahwa P, Karunanayake CP, Dosman JA, et al. Multiple myeloma and exposure to pesticides: a Canadian case-control study. J Agromedicine. 2012;17(1):40–50.
Presutti R, Harris SA, Kachuri L, et al. Pesticide exposures and the risk of multiple myeloma in men: an analysis of the North American Pooled Project. Int J Cancer. 2016;139(8):1703–14.
Figgs LW, Holland NT, Rothman N, et al. Increased lymphocyte replicative index following 2,4-dichlorophenoxyacetic acid herbicide exposure. Cancer Causes Control. 2000;11:373–80.
Lavrik IN, Golks A, Krammer PH, et al. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115(10):2665–72.
Raab MS, Podar K, Breitkreutz I, et al. Multiple myeloma. Lancet. 2009;374(9686):324–39.
Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110(2):125–8.
Randhawa N, Gulland F, Ylitalo GM, et al. Sentinel California sea lions provide insight into legacy organochlorine exposure trends and their association with cancer and infectious disease. One Health. 2015;1:37–43.
Li L, Zhang Y, Wang J, et al. History traces of HCHs and DDTs by groundwater dating and their behaviours and ecological risk in northeast China. Chemosphere. 2020;257:127212.
Mansouri A, Cregut M, Abbes C, et al. The environmental issues of DDT pollution and bioremediation: a multidisciplinary review. Appl Biochem Biotechnol. 2017;181:309–39.
Thakur M, Pathania D. Environmental fate of organic pollutants and effect on human health. In: Singh P, Kumar A, Borthakur A, editors. Abatement of environmental pollutants. Elsevier; 2020. p. 245–62.
Jaga K, Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int J Occup Med Environ Health. 2003;16(1):7–20.
John TJ, Dandona L, Sharma VP, et al. Continuing challenge of infectious diseases in India. Lancet. 2011;377(9761):252–69.
Van den Berg H. Global status of DDT and its alternatives for use in vector control to prevent disease. Environ Health Perspect. 2009;117(11):1656–63.
Garabrant DH, Held J, Langholz B, et al. DDT and related compounds and risk of pancreatic cancer. J Natl Cancer Inst. 1992;84(10):764–71.
Fryzek JP, Garabrant DH, Harlow SD, et al. A case-control study of self-reported exposures to pesticides and pancreas cancer in southeastern Michigan. Int J Cancer. 1997;72(1):62–7.
Cohn BA, Wolff MS, Cirillo PM, et al. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–14.
McGlynn KA, Quraishi SM, Graubard BI, et al. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst. 2008;100(9):663–71.
Persson EC, Graubard BI, Evans AA, et al. Dichlorodiphenyltrichloroethane and risk of hepatocellular carcinoma. Int J Cancer. 2012;131(9):2078–84.
Alavanja MC, Hofmann JN, Lynch CF, et al. Non-Hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS One. 2014;9(10):e109332.
Harada T, Takeda M, Kojima S, et al. Toxicity and carcinogenicity of dichlorodiphenyltrichloroethane (DDT). Toxicol Res. 2016;32(1):21–33.
Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2, 4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16(8):891–2.
Kelce WR, Stone CR, Laws SC, et al. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–5.
Stejskal V, Vendl T, Aulicky R, et al. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects. 2021;12(7):590.
Saadati N, Abdullah MP, Zakaria Z, et al. Distribution and fate of HCH isomers and DDT metabolites in a tropical environment–case study Cameron Highlands–Malaysia. Chem Cent J. 2012;6(130):1–15.
Humphreys EH, Janssen S, Heil A, et al. Outcomes of the California ban on pharmaceutical lindane: clinical and ecologic impacts. Environ Health Perspect. 2008;116(3):297–302.
Ward MH, Colt JS, Metayer C, et al. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117(6):1007–13.
Pattnaik M, Pany BK, Dena J, et al. Effect of organochlorine pesticides on living organisms and environment. Chem Sci Rev Lett. 2020;9:682–6.
Kasozi GN, Kiremire BT, Bugenyi FWB, et al. Organochlorine residues in fish and water samples from Lake Victoria, Uganda. J Environ Qual. 2006;35(2):584–9.
Parada H, Wolff MS, Engel LS, et al. Organochlorine insecticides DDT and chlordane in relation to survival following breast cancer. Int J Cancer. 2016;138(3):565–75.
Kachuri L, Beane Freeman LE, Spinelli JJ, et al. Insecticide use and risk of non-Hodgkin lymphoma subtypes: a subset meta-analysis of the North American Pooled Project. Int J Cancer. 2020;147(12):3370–83.
Lerro CC, Freeman LEB, DellaValle CT, et al. Pesticide exposure and incident thyroid cancer among male pesticide applicators in Agricultural Health Study. Environ Int. 2021;146:106187.
Mortazavi N, Asadikaram G, Ebadzadeh MR, et al. Organochlorine and organophosphorus pesticides and bladder cancer: a case-control study. J Cell Biochem. 2019;120(9):14847–59.
Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents Inflamm Allergy Drug Discov. 2009;3(1):73–80.
Breckenridge CB, Campbell JL, Clewell HJ, et al. PBPK-based probabilistic risk assessment for total chlorotriazines in drinking water. Toxicol Sci. 2016;150(2):269–82.
He H, Liu Y, You S, et al. A review on recent treatment technology for herbicide atrazine in contaminated environment. Int J Environ Res Public Health. 2019;16(24):5129.
Jones RM, Stayner LT, Demirtas H. Multiple imputation for assessment of exposures to drinking water contaminants: Evaluation with the Atrazine Monitoring Program. Environ Res. 2014;134:466–73.
Hopenhayn-Rich C, Stump ML, Browning SR. Regional assessment of atrazine exposure and incidence of breast and ovarian cancers in Kentucky. Arch Environ Contam Toxicol. 2002;42:127–36.
Inoue-Choi M, Weyer PJ, Jones RR, et al. Atrazine in public water supplies and risk of ovarian cancer among postmenopausal women in the Iowa Women’s Health Study. Occup Environ Med. 2016;73(9):582–7.
Rull RP, Gunier R, Von Behren J, et al. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ Res. 2009;109(7):891–9.
Malagoli C, Costanzini S, Heck JE, et al. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int J Hyg Environ Health. 2016;219(8):742–8.
McElroy JA, Gangnon RE, Newcomb PA, et al. Risk of breast cancer for women living in rural areas from adult exposure to atrazine from well water in Wisconsin. J Expo Sci Environ Epidemiol. 2007;17(2):207–14.
Tinfo NS, Hotchkiss MG, Buckalew AR, et al. Understanding the effects of atrazine on steroidogenesis in rat granulosa and H295R adrenal cortical carcinoma cells. Reprod Toxicol. 2011;31(2):184–93.
Urseler N, Bachetti R, Biolé F, et al. Atrazine pollution in groundwater and raw bovine milk: water quality, bioaccumulation and human risk assessment. Sci Total Environ. 2022;852:158498.
Sidhu GK, Singh S, Kumar V, et al. Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit Rev Environ Sci Technol. 2019;49(13):1135–87.
Mink PJ, Mandel JS, Sceurman BK, et al. Epidemiologic studies of glyphosate and cancer: a review. Regul Toxicol Pharmacol. 2012;63(3):440–52.
Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. 2016;28(1):1–15.
Davoren MJ, Schiestl RH. Glyphosate-based herbicides and cancer risk: a post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis. 2018;39(10):1207–15.
Samsel A, Seneff S. Glyphosate, pathways to modern diseases IV: cancer and related pathologies. J Biol Phys Chem. 2015;15(3):121–59.
Swanson NL, Leu A, Abrahamson J, et al. Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J Org Chem. 2014;9(2):6–37.
Alavanja MC, Sandler DP, McMaster SB, et al. The agricultural health study. Environ Health Perspect. 1996;104(4):362–9.
Engel LS, Hill DA, Hoppin JA, et al. Pesticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. Am J Epidemiol. 2005;161(2):121–35.
Lee WJ, Sandler DP, Blair A, et al. Pesticide use and colorectal cancer risk in the Agricultural Health Study. Int J Cancer. 2007;121(2):339–46.
Andreotti G, Beane Freeman LE, Hou L, et al. Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study Cohort. Int J Cancer. 2009;124(10):2495–500.
Dennis LK, Lynch CF, Sandler DP, et al. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect. 2010;118(6):812–7.
Andreotti G, Koutros S, Hofmann JN, et al. Glyphosate use and cancer incidence in the agricultural health study. J Natl Cancer Inst. 2018;110(5):509–16.
De Roos A, Zahm SH, Cantor KP, et al. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup Environ Med. 2003;60(9):e11.
Pahwa M, Beane Freeman LE, Spinelli JJ, et al. Glyphosate use and associations with non-Hodgkin lymphoma major histological sub-types. Scand J Work Environ Health. 2019;45(6):600–9.
Eriksson M, Hardell L, Carlberg M, et al. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 2008;123(7):1657–63.
Miller K. Estrogen and DNA damage: the silent source of breast cancer? J Natl Cancer Inst. 2003;95(2):100–2.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–7.
Solomon KR. Glyphosate in the general population and in applicators: a critical review of studies on exposures. Crit Rev Toxicol. 2016;46(sup1):21–7.
Bonner MR, Coble J, Blair A, et al. Malathion exposure and the incidence of cancer in the agricultural health study. Am J Epidemiol. 2007;166(9):1023–34.
Eddleston M, Karalliedde L, Buckley N, et al. Pesticide poisoning in the developing world—a minimum pesticides list. The Lancet. 2002;360(9340):1163–7.
Gilliom RJ, Barbash JE, Crawford CG et al. 2007. Pesticides in the nation’s streams and ground water 1992–2001. National Water Quality Assessment Program, Circular 1291. US Geological Survey. https://pubs.usgs.gov/circ/2005/1291/pdf/circ1291.pdf. Accessed 11 Feb 2023.
Koutros S, Harris SA, Spinelli JJ, et al. Non-Hodgkin lymphoma risk and organophosphate and carbamate insecticide use in the North American Pooled Project. Environ Int. 2019;127:199–205.
Latifovic L, Beane Freeman LE, Spinelli JJ, et al. Pesticide Use and Risk of Hodgkin Lymphoma: Results from the North American Pooled Project (NAPP). Cancer Causes Control. 2020;31:583–99.
Engel LS, Werder E, Satagopan J, et al. Insecticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. Environ Health Perspect. 2017;125(9):097002.
Echiburu-Chau C, Calaf GM. Rat lung cancer induced by malathion and estrogen. Int J Oncol. 2008;33(3):603–11.
Calaf GM, Bleak TC, Roy D. Signs of carcinogenicity induced by parathion, malathion, and estrogen in human breast epithelial cells. Oncol Rep. 2021;45(4):24.
Bradberry SM, Proudfoot AT, Vale JA. Poisoning due to chlorophenoxy herbicides. Toxicol Rev. 2004;23:65–73.
Peterson MA, McMaster SA, Riechers DE, et al. 2, 4-D past, present, and future: a review. Weed Technol. 2016;30(2):303–45.
Ozkul M, Ozel CA, Yuzbasioglu D, et al. Does 2, 4-dichlorophenoxyacetic acid (2, 4-D) induce genotoxic effects in tissue cultured Allium roots? Cytotechnology. 2016;68:2395–405.
Miligi L, Costantini AS, Veraldi A, et al. Cancer and pesticides: an overview and some results of the Italian multicenter case–control study on hematolymphopoietic malignancies. Ann N Y Acad Sci. 2006;1076(1):366–77.
Burns C, Bodner K, Swaen G, et al. Cancer incidence of 2, 4-D production workers. Int J Environ Res Public Health. 2011;8(9):3579–90.
Smith AM, Smith MT, La Merrill MA, et al. 2,4-Dichlorophenoxyacetic acid (2,4-D) and risk of non-Hodgkin lymphoma: a meta-analysis accounting for exposure levels. Ann Epidemiol. 2017;27(4):281–9.
Bukowska B. Effects of 2, 4-D and its metabolite 2, 4-dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(4):435–41.
Troudi A, Ben Amara I, Samet AM, et al. Oxidative stress induced by 2, 4-phenoxyacetic acid in liver of female rats and their progeny: Biochemical and histopathological studies. Environ Toxicol. 2012;27(3):137–45.
Palmeira CM, Moreno AJ, Madeira VM. Thiols metabolism is altered by the herbicides paraquat, dinoseb and 2, 4-D: a study in isolated hepatocytes. Toxicol Lett. 1995;81(2–3):115–23.
Bukowska B. Toxicity of 2, 4-dichlorophenoxyacetic acid--molecular mechanisms. Pol J Environ Stud. 2006;15(3):365–74.
Lerro CC, Hofmann JN, Andreotti G, et al. Dicamba use and cancer incidence in the Agricultural Health Study: an updated analysis. Int J Epidemiol. 2020;49(4):1326–37.
Alavanja MC, Dosemeci M, Samanic C, et al. Pesticides and lung cancer risk in the agricultural health study cohort. Am J Epidemiol. 2004;160(9):876–85.
Samanic C, Rusiecki J, Dosemeci M, et al. Cancer incidence among pesticide applicators exposed to dicamba in the agricultural health study. Environ Health Perspect. 2006;114(10):1521–6.
Leon ME, Schinasi LH, Lebailly P, et al. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int J Epidemiol. 2019;48(5):1519–35.
Mesnage R, Brandsma I, Moelijker N, et al. Genotoxicity evaluation of 2, 4-D, dicamba and glyphosate alone or in combination with cell reporter assays for DNA damage, oxidative stress and unfolded protein response. Food Chem Toxicol. 2021;157:112601.
Espandiari P, Glauert HP, Lee EY, et al. Promoting activity of the herbicide dicamba (2-methoxy-3, 6-dichlorobenzoic acid) in two stage hepatocarcinogenesis. Int J Oncol. 1999;14(1):79–163.
Lanasa MC, Weinberg JB. Immunologic aspects of monoclonal B-cell lymphocytosis. Immunol Res. 2011;49:269–80.
Thatheyus AJ, Selvam AG. Synthetic pyrethroids: toxicity and biodegradation. Appl Ecol Environ Sci. 2013;1(3):33–6.
Boulware DR, Beisang AA. Passive prophylaxis with permethrin-treated tents reduces mosquito bites among North American summer campers. Wilderness Environ Med. 2005;16(1):9–15.
Gupta R, Gahlot P, Purohit P. Pyrethroids: exposure, toxicity, and their influence on human health. J Environ Sci Health C Toxicol Carcinog. 2013;31(4):363–88.
Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117(4):581–6.
Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children< 2 years of age. Environ Health Perspect. 2013;121(2):269–75.
Boffetta P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48(6):433–42.
Shrestha S, Parks CG, Umbach DM, et al. Use of permethrin and other pyrethroids and mortality in the Agricultural Health Study. Occup Environ Med. 2022;79(10):664–72.
Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F436–7.
LaFiura KM, Bielawski DM, Posecion NC Jr, et al. Association between prenatal pesticide exposures and the generation of leukemia-associated T (8; 21). Pediatr Blood Cancer. 2007;49(5):624–8.
Ye M, Beach J, Martin JW, et al. Occupational pesticide exposures and respiratory health. Int J Environ Res Public Health. 2013;10(12):6442–71.
Paiga P, Morais S, Correia M, et al. Determination of carbamate and urea pesticide residues in fresh vegetables using microwave-assisted extraction and liquid chromatography. Int J Environ Anal Chem. 2009;89(3):199–210.
Wei G, Li Y, Wang X. Comparison of efficiencies between single-drop microextraction and continuous-flow microextraction for the determination of methomyl in natural waters. Int J Environ Anal Chem. 2008;88(6):397–408.
Çelebi MS, Oturan N, Zazou H, et al. Electrochemical oxidation of carbaryl on platinum and boron-doped diamond anodes using electro-Fenton technology. Sep Purif Technol. 2015;3:996–1002.
World Health Organization. 2006. Pesticides and their application: for the control of vectors and pests of public health importance. https://apps.who.int/iris/handle/10665/69223.
Pesatori AC, Sontag JM, Lubin JH, et al. Cohort mortality and nested case-control study of lung cancer among structural pest control workers in Florida (United States). Cancer Causes Control. 1994;5(4):310–8.
Mahajan R, Blair A, Coble J, et al. Carbaryl exposure and incident cancer in the Agricultural Health Study. Int J Cancer. 2007;121(8):1799–805.
Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113(25):6386–91.
Boccolini PD, Boccolini CS, Chrisman JD, et al. Non-Hodgkin lymphoma among Brazilian agricultural workers: a death certificate case-control study. Arch Environ Occup Health. 2017;72(3):139–44.
Acquavella JF, Alexander BH, Mandel JS, et al. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 2004;112(3):321–6.
Xia Y, Cheng S, Bian Q, et al. Genotoxic effects on spermatozoa of carbaryl-exposed workers. Toxicol Sci. 2005;85(1):615–23.
Peyre L, Zucchini-Pascal N, De Sousa G, et al. Potential involvement of chemicals in liver cancer progression: an alternative toxicological approach combining biomarkers and innovative technologies. Toxicol In Vitro. 2014;28(8):1507–20.
Alavanja MC. Pesticides use and exposure extensive worldwide. Environ Health Rev. 2009;24(4):303–9.
Damalas CA, Koutroubas SD. Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics. 2016;4(1):1.
Arcury TA, Grzywacz JG, Chen H, et al. Variation across the agricultural season in organophosphorus pesticide urinary metabolite levels for Latino farmworkers in eastern North Carolina: project design and descriptive results. Am J Ind Med. 2009;52(7):539–50.
Temkin AM, Uche UI, Evans S, et al. Racial and social disparities in Ventura County, California related to agricultural pesticide applications and toxicity. Sci Total Environ. 2022;853:158399.
Gee GC, Payne-Sturges DC, Martinez M. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2007;115(5):1645–55.
Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–32.
Quandt SA, Hernández-Valero MA, Grzywacz JG, et al. Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environ Health Perspect. 2006;114(6):943–52.
Zhang L, Rana I, Shaffer RM, et al. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: a meta-analysis and supporting evidence. Mutat Res Rev Mutat Res. 2019;781:186–206.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Roh, T., Aggarwal, A., Hasan, N.T., Upadhyay, A., Trisha, N.F. (2023). Pesticides and Cancer. In: Bernicker, E.H. (eds) Environmental Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-33750-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-33750-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33749-9
Online ISBN: 978-3-031-33750-5
eBook Packages: MedicineMedicine (R0)