Abstract
The activity of mining enterprises and mining and processing enterprises comes with the generation of wastewater of complex chemical composition in high volumes. Open-pit mining using ammonium nitrate leads to significant pollution of quarry and drainage waters with inorganic nitrogen compounds. Excessive content of inorganic nitrogen causes eutrophication of water bodies and afflicts significant damage to water resources. Biological methods used in treatment of nitrogen-containing wastewater depend significantly on external factors and show insufficiency in quarry wastewater treatment. The development of effective technologies based on physico-chemical treatment methods, in particular on the application of redox methods, is necessary for removing nitrogen compounds from mining wastewater. This paper presents the research results of the application of redox systems consisting of iron scraps and carbon-containing materials to reduce the content of nitrate ions in quarry water of mining enterprises. It was found that strong reducing agents Fe0, Fe2+, H2 as well as atomic hydrogen, capable of denitrifying water, are formed as a result of electrochemical processes in the redox system. The influence of the examined water pH and mass ratio of current-conducting elements in galvanic couple on efficiency of removing nitrate ions from quarry water was found. The possibility of post-treatment of quarry water, removing ammonium ions with the use of lowland peat, is shown.
Similar content being viewed by others
Keywords
1 Introduction
In the mining and processing industry, during open-pit mining through drilling and blasting operations, quarry and drainage waters are generated. The waters are polluted with inorganic nitrogen compounds (ammonium ions, nitrite and nitrate ions) that are products of decomposition and incomplete use of ammonium nitrate-based explosives [1, 2]. By some estimates, the average amount of NOx released into the environment when using ammonium nitrate-based explosives is 5 kg per 1 tonne of explosive [3]. Multiple exceedance of maximum allowable concentrations of nitrates, nitrites and ammonium ions in drainage water and effluent from mining and processing facilities is one of the challenging environmental and technological problems to solve [4,5,6,7,8].
Low content of organic impurities and bacterial microflora, which does not allow using traditional treatment technologies based on biological methods, are characteristic of quarry and drainage waters. When developing treatment technologies, it is necessary to consider that quarry and drainage waters generated volumes amount to millions of tonnes a year, and for their treatment it is economically expedient to apply cost efficient and effective methods.
Analysis of scientific and technical information showed that for removing nitrogen compounds from quarry water it is proposed to use bioengineered systems, in particular bio-plateau containing hydrophytes as well as microalgae or duckweeds [9, 10]. High activity of nitrate reductase, the enzyme that provides nitrate reduction in plant cells, is conditioned by high photosynthetic activity of plants [11].
The use of wetlands allows combining nitrate assimilation by plants and anaerobic denitrification in bottom layers [12]. However, the method of microbial denitrification depends significantly on transfer of electrons, which are donated by organic compounds in particular [13]. The deficit of electron donors makes the method of microbial denitrification inefficient in the treatment of wastewater with low content of organic substances, in particular quarry wastewater from mining enterprises.
Physico-chemical methods of quarry water treatment are based on creation of filtering sorption dams, masses, trenches with the use of natural materials as sorbents [14].
Currently, the possibility of using artificial chemical barriers containing redox systems for treatment of quarry waters is being investigated. For example, there are known studies of removing a wide range of pollutants (nitrate ions, chromate ions, etc.) from groundwater using zero-valent iron as a reducing agent, which acts as an electron donor [15, 16].
The mechanism of wastewater treatment using iron consists of direct reduction of nitrate and nitrite ions by metallic iron in a weakly acidic environment. Permeable reactive barrier is put into effect in the form of a subsurface placement of chemically active raw materials. The pollutant flow passes under the natural gradient through the barrier material. The PRB system is implemented in situ, completely passively, which allows it to be operated for a long time with minimal maintenance [17, 18].
Known as the galvanochemical method (galvanocoagulation), it is used for wastewater treatment to remove fine particles, colloidal impurities, heavy metal ions, oxidant ions, such as chromate ions. The galvanocoagulation method is based on the principle of a short-circuited galvanic element, in which the water is treated with a mixture of conducting materials, one of which has a high reducing capacity. The current density in galvanic couple field will depend on the materials of the cathode and anode sections [19, 20].
The purpose of this work was to evaluate the effectiveness of zero-valent iron as well as galvanic system, containing iron scraps and carbon materials, in reducing the content of nitrate ions in quarry wastewater of mining and processing enterprises.
2 Materials and Methods
The subject of the study was the quarry wastewater sampled from the quarry water settling tank of a large mining and processing enterprise in Russia.
In accredited laboratory, the chemical composition of the examined wastewater was analyzed using standard methods (the content of nitrate ions was determined photometrically in accordance with PND F 14.1:2:4.4-95, the content of ammonium ions was determined by capillary electrophoresis according to GOST 31869-2012).
We studied the process of nitrate ions reduction by zero-valent iron, for which iron scraps were used, and made a model of nitrate ions reduction by a redox system consisting of galvanic couple “iron scraps-active carbon production waste”. The influence of Fe:C mass ratio and treatment time on the efficiency of removing nitrate ions from the water was examined.
The process was controlled by the residual content of nitrate ions in the studied water.
In order to activate the iron oxidation processes, it is advisable to conduct the process in a weakly acidic environment. For this purpose, we treated current-generating materials with an acid solution for a day at pH = 4.0–4.5. After that, we added the water to the container and carried out the purification process while stirring the suspension.
The effect of pH on the rate and completeness of nitrate ions reduction was studied. The initial pH value of wastewater was set using HCl and NaOH solutions, the pH value was determined potentiometrically. Upon completing the denitrification process, the pH was measured again.
It is known that quarry waters also contain ammonium ions in amounts significantly exceeding the maximum allowable concentrations. The paper presents the research results of the extraction of ammonium ions by lowland peat samples. To determine the sorption capacity of peat by ammonium ions in static conditions, we treated dry peat sub-samples weighing 5 g with 50 ml of wastewater while stirring until equilibration. Studies were conducted at pH 6 and 8. To achieve an alkaline reaction of the medium, CaO was added to the sample.
3 Results and Discussion
3.1 Chemical Composition of Quarry Waters
The composition of the studied wastewater sampled from the quarry water settling tank of the mining and processing enterprise is presented in Table 1.
It was found that the examined quarry wastewater had high content of nitrate ions (204 mg/dm3), ammonium ions (9.7 mg/dm3) and exceeded amount of manganese ions. The obtained results of the quarry water analysis are in agreement with the literature data [21,22,23].
3.2 Use of Redox Systems for Removing Nitrate Ions from Quarry Water
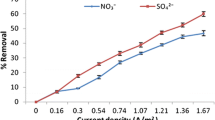
Figures 1 and 2 show the obtained research results of removing nitrate ions from quarry water using zero-valent iron and galvanic couple at the initial value of the water pH 6 (Fig. 1) and at pH 4 (Fig. 2).
It was found that the effectiveness of removing nitrate ions from the water is significantly affected by pH value. When treating the water in acidic environment, the purification efficiency significantly increases and treatment time reduces. Purification efficiency with the use of galvanic couple, when the Fe:C ratio is 3:1, reaches 90% with a contact time of 3 h.
When using zero-valent iron under these conditions, the purification efficiency is 50%. The lower effect can be explained by different mechanisms of nitrate ions reduction by the studied systems.
When using zero-valent iron, the process consists of direct reduction of nitrate ions by iron [24, 25]:
To increase the integrity of the reactions, it is necessary to maintain acidity of the medium.
Denitrification of quarry waters using redox system consisting of iron scraps and carbon-containing materials is studied for the first time.
The mechanism of galvanochemical water purification (galvanocoagulation) is based on electrochemical processes. Due to the difference of electrochemical potentials of current-conducting elements, on the contact Fe0 - carbon-containing material many galvanic couples appear, which causes intensive oxidation and dissolution of metal, electrolysis of water, pH shift, hydrolysis and other physical and chemical processes.
At the anode areas of galvanic couples, Fe0 is oxidized and the following reactions are possible:
Anode Area – Fe
The resulting ions are able to undergo hydrolysis according to the following scheme:
The resulting Fe2+ ions and iron (II) hydroxide can be oxidized by water-dissolved oxygen or nitrate ions:
The cathode areas of galvanic couples (carbon-containing material) are cathodically polarized, and the following reactions are possible in the presence of oxygen:
In an acidic environment:
Hydrogen released at the cathode areas can also take part in the nitrate ion reduction:
Hydrolysis of Fe2+ and Fe3+ ions comes with a decrease in the pH value; at the same time, the hydroxide ion will be released at the cathode areas in weakly acidic and neutral environments, which allows maintaining the pH value at 6–7.
Thus, as a result of electrochemical processes, strong reducing agents Fe0, Fe2+, H2 as well as atomic hydrogen are formed in the redox system “iron scraps- carbon”, which leads to significant increase in the efficiency of nitrate ions removal from the water compared with the use of zero-valent iron for this purpose.
3.3 Deammonification of Quarry Wastewater
In order to study the possibility of deammonification of effluents, the use of peat was studied. It is known that peat can reduce the concentration of ammonium ions due to both the porous structure and the interaction of humic acids in its composition.
We used samples of peat with natural pH value and samples pre-treated with calcium oxide. A number of authors [26] cite data on the effect of pH on the binding of heavy metal ions by humic acids of peats. The authors point out that the sorption of heavy metals increases with increasing pH. In such a case, not only hydrogen of carboxyl groups is replaced by metal ions located on the surface of the humic substances molecule, but also the sorption and sedimentation of metal ions on already formed insoluble humate (secondary sorption) takes place [26].
It was found that at pH 6 the purification efficiency of the studied ammonium ions solution was 93%; at pH 8 the purification efficiency reached 98%. Thus, the experimental data obtained also agree with the aforementioned studies and show that ammonium ions formed as a result of nitrate ions reduction can be removed from wastewater using natural material - peat.
4 Conclusion
The main source of nitrate ions and ammonium ions pollution of drainage and quarry waters of mining enterprises during mining by open-pit drilling and blasting method is the use of explosives containing ammonium nitrate. Typical features of quarry wastewaters are their high volumes and low content of organic impurities, which complicates natural mechanisms of denitrification and determines the complexity of the choice of methods for nitrogen compounds removal from mining wastewater. Significant volumes of wastewater make it economically feasible to use fairly inexpensive but effective methods of denitrification.
The obtained experimental data indicate the possibility to apply redox systems containing the galvanic couple “iron scraps - active carbon production wastes” for treating high volumes of wastewater from mining enterprises. To reduce the concentration of ammonium ions in wastewater after its treatment using galvanochemical method (galvanocoagulation), lowland peat, which has high sorption and chemisorption activity against ammonium ions in slightly alkaline environment, can be used.
Implementation of the studied method of removing nitrate ions and ammonium ions from the water is possible by creating a geochemical permeable barrier containing galvanic couple, sand and peat.
References
Ferreira, H., Garcia Praça Leite, M.: A life cycle assessment study of iron ore mining. J. Clean. Product. 108(A), 1081–1091 (2015). https://doi.org/10.1016/j.jclepro.2015.05.140
Studenok, A., Studenok, G., Revvo, A.: Evaluation of wastewater treatment methods from nitrogen compounds for drainage waters of mining enterprises. News Ural State Min. Univ. 2(30), 26–30 (2013)
Oluwoye, I., Dlugogorski, B., Gore, J., Oskierski, H., Altarawneh, M.: Atmospheric emission of NOx from mining explosives: a critical review. Atmos. Environ. 167, 81–96 (2017). https://doi.org/10.1016/j.atmosenv.2017.08.006
Liu, Y., Li, H., An, H., Guan, J., Shi, J., Han, X.: Are the environmental impacts, resource flows and economic benefits proportional? Analysis of key global trade routes based on the steel life cycle. Ecol. Ind. 122, 107306 (2021). https://doi.org/10.1016/j.ecolind.2020.107306
Becker, F., Eser, R., Hoelzmann, P., Schütt, B.: The environmental impact of ancient iron mining and smelting on Elba Island, Italy – a geochemical soil survey of the Magazzini site. J. Geochem. Explor. 205, 106307 (2019). https://doi.org/10.1016/j.gexplo.2019.04.009
Sutherland, K.: Mining: filtration prospects for the iron ore mining industry. Filtrat. Separat. 51(6), 29–32 (2014). https://doi.org/10.1016/S0015-1882(14)70225-3
Sonter, L., Moran, C., Barrett, D., Soares-Filho, B.: Processes of land use change in mining regions. J. Clean. Prod. 84, 494–501 (2014). https://doi.org/10.1016/j.jclepro.2014.03.084
Jiskani, I., Cai, Q., Zhou, W., Ali Shah, S.: Green and climate-smart mining: a framework to analyze open-pit mines for cleaner mineral production. Resour. Policy 71, 102007 (2021). https://doi.org/10.1016/j.resourpol.2021.102007
Wang, J., et al.: A constructed wetland system with aquatic macrophytes for cleaning contaminated runoff/storm water from urban area in Florida. Journal of Environmental Management 280, 111794 (2021). https://doi.org/10.1016/j.jenvman.2020.111794
Li, X., Wu, S., Yang, C., Zeng, G.: Microalgal and duckweed based constructed wetlands for swine wastewater treatment: a review. Biores. Technol. 318, 123858 (2020). https://doi.org/10.1016/j.biortech.2020.123858
Wang, T., et al.: Nitrogen removal from summer to winter in a field pilot-scale multistage constructed wetland-pond system. J. Environ. Sci. 111, 249–262 (2022). https://doi.org/10.1016/j.jes.2021.03.028
Wang, X., et al.: Improving denitrification efficiency in constructed wetlands integrated with immobilized bacteria under high saline conditions. Environ. Pollut. 287, 117592 (2021). https://doi.org/10.1016/j.envpol.2021.117592
Ma, Y., Zheng, X., Fang, Y., Xu, K., He, S., Zhao, M.: Autotrophic denitrification in constructed wetlands: achievements and challenges. Biores. Technol. 318, 123778 (2020). https://doi.org/10.1016/j.biortech.2020.123778
Vodjanickij, J., Shoba, S.: Biogeochemical barriers for soil remediation and soil-groundwater treatment Vestnik Moskovskogo universiteta. Serija 17. Pochvovedenie 3, 3–15 (2016)
Zingaretti, D., Verginelli, I., Luisetto, I., Baciocchi, R.: Horizontal permeable reactive barriers with zero-valent iron for preventing upward diffusion of chlorinated solvent vapors in the unsaturated zone. J. Contam. Hydrol. 234, 103687 (2020). https://doi.org/10.1016/j.jconhyd.2020.103687
Maamoun, I., Eljamal, O., Falyouna, O., Eljamal, R., Sugihara, Y.: Multi-objective optimization of permeable reactive barrier design for Cr(VI) removal from groundwater. Ecotoxicol. Environ. Saf. 200, 110773 (2020). https://doi.org/10.1016/j.ecoenv.2020.110773
Franklin, O.-N., Johana Grajales-Mesa, S., Grzegorz, M.: An overview of permeable re-active barriers for in situ sustainable groundwater remediation. Chemosphere 111, 243–259 (2014). https://doi.org/10.1016/j.chemosphere.2014.03.112
Ona-Nguema, G., Guerbois, D., Pallud, C., Brest, J., Abdelmoula, M., Morin, G.: Biogenic Fe (II-III) hydroxycarbonate green rust enhances nitrate removal and decreases ammo-nium selectivity during heterotrophic denitrification. Minerals 10, 818 (2020). https://doi.org/10.3390/min10090818
Chanturija, V.A., Solozhenkin, P.M.: Galvanochemical methods of industrial water puri-fication: Theory and practice. Akademkniga, Moscow (2005). (in Russian)
Lugo-Lugo, V., Barrera-Díaz, C., Bilyeu, B., Balderas-Hernández, P., Ureña-Nuñez, F., Sánchez-Mendieta, V.: Cr(VI) reduction in wastewater using a bimetallic galvanic reactor. J. Hazard. Mater. 176(1–3), 418–425 (2010). https://doi.org/10.1016/j.jhazmat.2009.11.046
Mhlongo, S., Mativenga, P., Marnewick, A.: Water quality in a mining and water-stressed region. J. Clean. Prod. 171, 446–456 (2018). https://doi.org/10.1016/j.jclepro.2017.10.030
Blanchette, M., Lund, M.: Pit lakes are a global legacy of mining: an integrated approach to achieving sustainable ecosystems and value for communities. Curr. Opin. Environ. Sustain. 23, 28–34 (2016). https://doi.org/10.1016/j.cosust.2016.11.012
Northey, S., Mudd, G., Saarivuori, E., Wessman-Jääskeläinen, H., Haque, N.: Water footprinting and mining: Where are the limitations and opportunities? J. Clean. Prod. 135, 1098–1116 (2016). https://doi.org/10.1016/j.jclepro.2016.07.024
Huang, C., Wang, H., Chiu, P.: Nitrate reduction by metallic iron. Water Res. 32(8), 2257–2264 (1998). https://doi.org/10.1016/S0043-1354(97)00464-8
Huang, Y., Zhang, T.: Effects of low pH on nitrate reduction by iron powder. Water Res. 38(11), 2631–2642 (2004). https://doi.org/10.1016/j.watres.2004.03.015
Dmitrieva, E., Syundyukova, M., Leont’eva, M., Glebov, N.: Effect of pH on the binding of heavy metal ions by humic substances and hymatomelanic acids in peat, Uchenye Zapiski Kazanskogo Universiteta. Seriya Estestvennye Nauki 159(4), 575–588 (2017)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Glushankova, I.S., Bessonova, E.N., Blinov, S.M., Kudryashova, E.N., Belkin, P.A., Rudakova, L.V. (2022). Denitrification of Quarry Wastewater from Mining Enterprises by Galvanocoagulation. In: Rocha, A., Isaeva, E. (eds) Science and Global Challenges of the 21st Century - Science and Technology. Perm Forum 2021. Lecture Notes in Networks and Systems, vol 342. Springer, Cham. https://doi.org/10.1007/978-3-030-89477-1_34
Download citation
DOI: https://doi.org/10.1007/978-3-030-89477-1_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89476-4
Online ISBN: 978-3-030-89477-1
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)