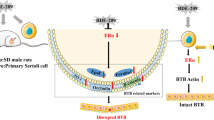
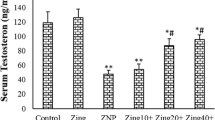
Abstract
The testis is one of the organs in the mammalian body that is sensitive to toxicants. Accumulating evidence has shown that human exposure to toxic ingredients in Traditional Chinese Medicine (TCM), such as triptolide, gossypol, cannabidol, piperine, α-solanine, matrine, aristolochic acid, and emodin, lead to testis injury and reproductive dysfunction. The most obvious phenotype is reduced sperm counts due to defects in spermatogenesis. Studies have also shown that Sertoli cells in the seminiferous tubule, the functional unit of the testis that supports spermatogenesis, are the cell type that is most sensitive to the disruptive effects of toxicants. Since Sertoli cells are the “mother cells” that nurture germ cell development, Sertoli cell injury thus leads to failure in germ cell development in the seminiferous epithelium. Mounting evidence has shown that the Sertoli cell cytoskeletons, mitochondria function, Leydig cells steroidogenesis pathways and sperm ion channels are some of the prime targets of toxicants from TCM. We carefully evaluate recent findings in this area of research herein, and to provide a summary of these findings, including some insightful information regarding the underlying molecular basis of toxicant-induced testis injury that impede spermatogenesis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Carney, E. F. (2014). Antiproteinuric efficacy of A. Manihot superior to losartan. Nature Reviews Nephrology, 10, 300.
Martel, J., Ojcius, D. M., Chang, C.-J., Lin, C.-S., Lu, C.-C., Ko, Y.-F., Tseng, S.-F., Lai, H.-C., & Young, J. D. (2016). Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nature Reviews Endocrinology, 13, 149.
Xiong, X. (2015). Integrating traditional Chinese medicine into Western cardiovascular medicine: An evidence-based approach. Nature Reviews Cardiology, 12, 374–374.
Yuan, H., Ma, Q., Ye, L., & Piao, G. (2016). The traditional medicine and modern medicine from natural products. Molecules, 21.
Wang, X., Zhao, F., Lv, Z. M., Shi, W. Q., Zhang, L. Y., & Yan, M. (2016). Triptolide disrupts the actin-based Sertoli-germ cells Adherens junctions by inhibiting rho Gtpases expression. Toxicology and Applied Pharmacology, 310, 32–40.
Ma, B., Qi, H., Li, J., Xu, H., Chi, B., Zhu, J., Yu, L., An, G., & Zhang, Q. (2015). Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (Ppar) levels in male mice testes followed by testicular injury: A Gc-Ms based metabolomics study. Toxicology, 336, 84–95.
Cheng, Y., Chen, G., Wang, L., Kong, J., Pan, J., Xi, Y., Shen, F., & Huang, Z. (2018). Triptolide-induced mitochondrial damage dysregulates fatty acid metabolism in mouse Sertoli cells. Toxicology Letters, 292, 136–150.
Jing, X., Cheng, W., Guo, S., Zou, Y., Zhang, T., & He, L. (2017). Toxic effects of Tripterygium Wilfordii hook F on the reproductive system of adolescent male rats. Biomedicine & Pharmacotherapy, 95, 1338–1345.
Zhou, D. R., Zhou, Y. C., Cui, G. H., Guo, X., Qin, J., Gui, Y. T., & Cai, Z. M. (2008). Gossypol repressed the gap junctional intercellular communication between Sertoli cells by decreasing the expression of Connexin43. Toxicology In Vitro, 22, 1719–1725.
Lim, W., Ham, J., Park, S., Bae, H., You, S., & Song, G. (2019). Gossypol induces disruption of spermatogenesis and steroidogenesis in male mice. Journal of Agricultural and Food Chemistry, 67, 2075–2085.
Carvalho, R. K., Santos, M. L., Souza, M. R., Rocha, T. L., Guimaraes, F. S., Anselmo-Franci, J. A., & Mazaro-Costa, R. (2018b). Chronic exposure to Cannabidiol induces reproductive toxicity in male Swiss mice. Journal of Applied Toxicology, 38, 1215–1223.
Carvalho, R. K., Souza, M. R., Santos, M. L., Guimaraes, F. S., Pobbe, R. L. H., Andersen, M. L., & Mazaro-Costa, R. (2018c). Chronic Cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reproductive Toxicology, 81, 34–40.
Chinta, G., Coumar, M. S., & Periyasamy, L. (2017). Reversible testicular toxicity of Piperine on male albino rats. Pharmacognosy Magazine, 13, S525–S532.
Chen, X., Ge, F., Liu, J., Bao, S., Chen, Y., Li, D., Li, Y., Huang, T., Chen, X., Zhu, Q., Lian, Q., & Ge, R. S. (2018). Diverged effects of Piperine on testicular development: Stimulating Leydig cell development but inhibiting spermatogenesis in rats. Frontiers in Pharmacology, 9, 244.
Park, S., Park, M. Y., Song, G., & Lim, W. (2019). Alpha-Solanine inhibits cell proliferation via mitochondrial dysfunction and inhibin synthesis in mouse testis in vitro and in vivo. Chemosphere, 235, 271–279.
Luo, T., Zou, Q. X., He, Y. Q., Wang, H. F., Wang, T., Liu, M., Chen, Y., & Wang, B. (2016). Matrine compromises mouse sperm functions by a [ca(2+)]I-related mechanism. Reproductive Toxicology, 60, 69–75.
Kwak, D. H., & Lee, S. (2016). Aristolochic acid I causes testis toxicity by inhibiting Akt and Erk1/2 phosphorylation. Chemical Research in Toxicology, 29, 117–124.
Cui, Y., Han, J., Ren, J., Chen, H., Xu, B., Song, N., Li, H., Liang, A., & Shen, G. (2019). Untargeted Lc-Ms-based Metabonomics revealed that Aristolochic acid I induces testicular toxicity by inhibiting amino acids metabolism, glucose metabolism, Beta-oxidation of fatty acids and the Tca cycle in male mice. Toxicology and Applied Pharmacology, 373, 26–38.
Oshida, K., Hirakata, M., Maeda, A., Miyoshi, T., & Miyamoto, Y. (2011). Toxicological effect of Emodin in mouse testicular gene expression profile. Journal of Applied Toxicology, 31, 790–800.
Luo, T., Li, N., He, Y. Q., Weng, S. Q., Wang, T., Zou, Q. X., & Zeng, X. H. (2015). Emodin inhibits human sperm functions by reducing sperm [ca(2+)]I and tyrosine phosphorylation. Reproductive Toxicology, 51, 14–21.
Goldbach-Mansky, R., Wilson, M., Fleischmann, R., Olsen, N., Silverfield, J., Kempf, P., Kivitz, A., Sherrer, Y., Pucino, F., Csako, G., Costello, R., Pham, T. H., Snyder, C., Van Der Heijde, D., Tao, X., Wesley, R., & Lipsky, P. E. (2009). Comparison of Tripterygium Wilfordii hook F versus sulfasalazine in the treatment of rheumatoid arthritis: A randomized trial. Annals of Internal Medicine, 151(229–40), W49–W51.
Wang, Y., Huang, L. Q., Tang, X. C., & Zhang, H. Y. (2010). Retrospect and Prospect of active principles from Chinese herbs in the treatment of dementia. Acta Pharmacologica Sinica, 31, 649–664.
Noel, P., Von Hoff, D. D., Saluja, A. K., Velagapudi, M., Borazanci, E., & Han, H. (2019). Triptolide and its derivatives as Cancer therapies. Trends in Pharmacological Sciences, 40, 327–341.
Huynh, P. N., Hikim, A. P., Wang, C., Stefonovic, K., Lue, Y. H., Leung, A., Atienza, V., Baravarian, S., Reutrakul, V., & Swerdloff, R. S. (2000). Long-term effects of Triptolide on spermatogenesis, Epididymal sperm function, and fertility in male rats. Journal of Andrology, 21, 689–699.
Ni, B., Jiang, Z., Huang, X., Xu, F., Zhang, R., Zhang, Z., Tian, Y., Wang, T., Zhu, T., Liu, J., & Zhang, L. (2008). Male reproductive toxicity and Toxicokinetics of Triptolide in rats. Arzneimittel-Forschung, 58, 673–680.
Grima, J., Wong, C. C., Zhu, L. J., Zong, S. D., & Cheng, C. Y. (1998). Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. The Journal of Biological Chemistry, 273, 21040–21053.
Grima, J., Zhu, L., & Cheng, C. Y. (1997). Testin is tightly associated with testicular cell membrane upon its secretion by Sertoli cells whose steady-state Mrna level in the testis correlates with the turnover and integrity of inter-testicular cell junctions. The Journal of Biological Chemistry, 272, 6499–6509.
Bao, J., & Dai, S. M. (2011). A Chinese herb Tripterygium Wilfordii hook F in the treatment of rheumatoid arthritis: Mechanism, efficacy, and safety. Rheumatology International, 31, 1123–1129.
Huang, M., Zhang, H., Liu, T., Tian, D., Gu, L., & Zhou, M. (2013). Triptolide inhibits Mdm2 and induces apoptosis in acute lymphoblastic leukemia cells through a P53-independent pathway. Molecular Cancer Therapeutics, 12, 184–194.
Jiao, J., Tang, X. P., Yuan, J., Liu, X., Liu, H., Zhang, C. Y., Wang, L. Y., & Jiang, Q. (2016). Effect of external applying compound Tripterygium Wilfordii hook F. on joint pain of rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi, 36, 29–34.
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R., & Ozcan, U. (2015). Treatment of obesity with Celastrol. Cell, 161, 999–1011.
Zhao, J., Di, T., Wang, Y., Liu, X., Liang, D., Zhang, G., & Li, P. (2016). Multi-glycoside of Tripterygium Wilfordii hook. F. Ameliorates Imiquimod-induced skin lesions through a Stat3-dependent mechanism involving the inhibition of Th17-mediated inflammatory responses. International Journal of Molecular Medicine, 38, 747–757.
Zheng, Y., Zhang, W. J., & Wang, X. M. (2013). Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neuroscience & Therapeutics, 19, 76–82.
Gao, Y., Chen, H., Xiao, X., Lui, W. Y., Lee, W. M., Mruk, D. D., & Cheng, C. Y. (2017). Perfluorooctanesulfonate (Pfos)-induced Sertoli cell injury through a disruption of F-actin and microtubule organization is mediated by Akt1/2. Scientific Reports, 7, 1110.
Chen, H., Gao, Y., Mruk, D. D., Xiao, X., John, C. M., Turek, P. J., Lui, W. Y., Lee, W. M., Silvestrini, B., & Cheng, C. Y. (2017). Rescue of Pfos-induced human Sertoli cell injury by overexpressing a P-Fak-Y407e Phosphomimetic mutant. Scientific Reports, 7, 15810.
Keshmiri-Neghab, H., & Goliaei, B. (2014). Therapeutic potential of gossypol: An overview. Pharmaceutical Biology, 52, 124–128.
Yuan, C., Song, H. H., Zhang, X. Y., Jiang, Y. J., Zhang, A. T., Azzam, M. M., & Zou, X. T. (2014). Effect of expanded cottonseed meal on laying performance, egg quality, concentrations of free gossypol in tissue, serum and egg of laying hens. Animal Science Journal, 85, 549–554.
Fiorini, C., Tilloy-Ellul, A., Chevalier, S., Charuel, C., & Pointis, G. (2004). Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reproductive Toxicology, 18, 413–421.
Herve, J. C., Pluciennik, F., Bastide, B., Cronier, L., Verrecchia, F., Malassine, A., Joffre, M., & Deleze, J. (1996). Contraceptive gossypol blocks cell-to-cell communication in human and rat cells. European Journal of Pharmacology, 313, 243–255.
Wan, H. T., Mruk, D. D., Wong, C. K. C., & Cheng, C. Y. (2014). Perfluorooctanesulfonate (Pfos) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via P-Fak-Tyr407 - An In Vitro study. Endocrinology, 155, 249–262.
Li, N., Mruk, D. D., Mok, K. W., Li, M. W., Wong, C. K., Lee, W. M., Han, D., Silvestrini, B., & Cheng, C. Y. (2016). Connexin 43 reboots meiosis and reseals blood-testis barrier following toxicant-mediated Aspermatogenesis and barrier disruption. The FASEB Journal, 30, 1436–1452.
Zhang, S., Mo, J., Wang, Y., Ni, C., Li, X., Zhu, Q., & Ge, R. S. (2019). Endocrine disruptors of inhibiting testicular 3beta-Hydroxysteroid dehydrogenase. Chemico-Biological Interactions, 303, 90–97.
Lin, T., Murono, E. P., Osterman, J., Nankin, H. R., & Coulson, P. B. (1981). Gossypol inhibits testicular steroidogenesis. Fertility and Sterility, 35, 563–566.
Qian, S.-Z. (1985). Gossypol-Hypokalaemia interrelationships. International Journal of Andrology, 8, 313–324.
Waites, G. M., Wang, C., & Griffin, P. D. (1998). Gossypol: Reasons for its failure to be accepted as a safe, reversible male antifertility drug. International Journal of Andrology, 21, 8–12.
Pisanti, S., Malfitano, A. M., Ciaglia, E., Lamberti, A., Ranieri, R., Cuomo, G., Abate, M., Faggiana, G., Proto, M. C., Fiore, D., Laezza, C., & Bifulco, M. (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacology & Therapeutics, 175, 133–150.
Brand, E. J., & Zhao, Z. (2017). Cannabis in Chinese medicine: Are some traditional indications referenced in ancient literature related to cannabinoids? Frontiers in Pharmacology, 8, 108.
Carvalho, R. K., Santos, M. L., Souza, M. R., Rocha, T. L., Guimaraes, F. S., Anselmo-Franci, J. A., & Mazaro-Costa, R. (2018a). Chronic exposure to Cannabidiol induces reproductive toxicity in male Swiss mice. Journal of Applied Toxicology, 38, 1545.
Srinivasan, K. (2007). Black pepper and its pungent principle-Piperine: A review of diverse physiological effects. Critical Reviews in Food Science and Nutrition, 47, 735–748.
Chinta, G., Ramya Chandar Charles, M., Klopcic, I., Sollner Dolenc, M., Periyasamy, L., & Selvaraj Coumar, M. (2015). In silico and in vitro investigation of the Piperine's male contraceptive effect: Docking and molecular dynamics simulation studies in androgen-binding protein and androgen receptor. Planta Medica, 81, 804–812.
Hasanain, M., Bhattacharjee, A., Pandey, P., Ashraf, R., Singh, N., Sharma, S., Vishwakarma, A. L., Datta, D., Mitra, K., & Sarkar, J. (2015). Alpha-Solanine induces Ros-mediated autophagy through activation of endoplasmic reticulum stress and inhibition of Akt/Mtor pathway. Cell Death & Disease, 6, E1860.
Rashid, H. U., Xu, Y., Muhammad, Y., Wang, L., & Jiang, J. (2019). Research advances on anticancer activities of Matrine and its derivatives: An updated overview. European Journal of Medicinal Chemistry, 161, 205–238.
Zhang, S., Zhang, Y., Zhuang, Y., Wang, J., Ye, J., Zhang, S., Wu, J., Yu, K., & Han, Y. (2012). Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One, 7, E46853.
Yong, J., Wu, X., & Lu, C. (2015). Anticancer advances of Matrine and its derivatives. Current Pharmaceutical Design, 21, 3673–3680.
Pu, X. Y., Shen, J. Y., Deng, Z. P., & Zhang, Z. A. (2017). Plasma-specific Microrna response induced by acute exposure to Aristolochic acid I in rats. Archives of Toxicology, 91, 1473–1483.
Dong, X., Fu, J., Yin, X., Cao, S., Li, X., Lin, L., & Huyiligeqi & Ni, J. (2016). Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytotherapy Research, 30, 1207–1218.
Srinivas, G., Babykutty, S., Sathiadevan, P. P., & Srinivas, P. (2007). Molecular mechanism of Emodin action: Transition from laxative ingredient to An antitumor agent. Medicinal Research Reviews, 27, 591–608.
Acknowledgements
This work was supported in part by a grant from the Wenzhou Medical University (Wenzhou, Zhejiang, China) to C.Y.C.; L.W. was supported by a China Pharmaceutical University World Explorer Study Abroad Scholarship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yan, M., Wang, L., Cheng, C.Y. (2021). Testis Toxicants: Lesson from Traditional Chinese Medicine (TCM). In: Cheng, C., Sun, F. (eds) Molecular Mechanisms in Spermatogenesis. Advances in Experimental Medicine and Biology, vol 1381. Springer, Cham. https://doi.org/10.1007/978-3-030-77779-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-77779-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77778-4
Online ISBN: 978-3-030-77779-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)