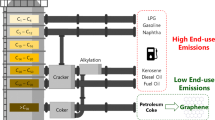
Abstract
The low atomic number of carbons, combined with the half-full shell of valence electrons and medium electronegativity, provide an important basis for strong covalent bonding to other carbon atoms and other elements. Moreover, this supports the wide differences of carbon-containing natural atoms, counting the atoms of life. Recent breakthroughs in carbon-based nanomaterials’ science and technology use paraffinic waxes as a carbon source where it consists of not less than 18 carbon number per single paraffin crystal. Paraffinic waxes are considered a cheap by-product in the petroleum refinery, which is considered a source for nanocarbon synthesis, whether it is activated nanocarbon or carbon nanotubes and nanocarbon fibers. This chapter describes the separation of paraffinic petroleum wax, its purification, and characterization, besides that, the synthesis of nanocarbon and its evaluation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ACs:
-
Activated carbons
- CNTs:
-
Carbon nanotubes
- CVD:
-
Chemical vapor deposition
- EPA:
-
Environmental protection agency
- GO:
-
Graphene oxide
- MWCNTs:
-
Multi wall carbon nanotubes
- RGO:
-
Reduced graphene oxide
- SWCNTs:
-
Single wall carbon nanotubes
- TNT:
-
Titanium nanotube
- UV:
-
Ultra-violet
References
Awadallah AE, Aboul-Enein AA, Aboul-Gheit AK (2014) Impact of group VI metals addition to Co/MgO catalyst for non-oxidative decomposition of methane into COx-free hydrogen and carbon nanotubes. Fuel 129:27–36
Awadallah AE, Aboul-Enein AA, Aboul-Gheit AK (2014) Effect of progressive Co loading on commercial Co–Mo/Al2O3 catalyst for natural gas decomposition to COx-free hydrogen production and carbon nanotubes. Energy Convers Manage 77:143–151
Li J, Dong L, Xiong L, Yang Y, Du Y, Zhao L, Wang H, Peng S (2016) High-loaded NiCuSiO2 catalysts for methane decomposition to prepare hydrogen and carbon filaments. Int J Hydrogen Energy 41(28):12038–12048
Li Y, Li D, Wang G (2011) Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: a review. Catal Today 162(1):1–48
Aboul-Enein AA, Soliman FS, Betiha MA (2019) Co-production of hydrogen and carbon nanomaterials using NiCu/SBA15 catalysts by pyrolysis of a wax by-product: effect of Ni–Cu loading on the catalytic activity. Int J Hydrogen Energy 44(59):31104–31120
Thomas R (2008) Process chemistry of lubricant base stocks: conventional base stock production. CRC Press, London
Mazee W (1973) Modern petroleum technology. Applied Science Publishers Ltd., on behalf of The Institute of Petroleum, Great Britain, p 782
Sharma R, Monga Y, Puri A (2014) Magnetically separable silica@Fe3O4 core–shell supported nano-structured copper (II) composites as a versatile catalyst for the reduction of nitroarenes in aqueous medium at room temperature. J Mol Catal A: Chem 393:84–95
Sharifi A, Montazerghaem L, Naeimi A, Abhari AR, Vafaee M, Ali GAM, Sadegh H (2019) Investigation of photocatalytic behavior of modified ZnS:Mn/MWCNTs nanocomposite for organic pollutants effective photodegradation. J Environ Manage 247:624–632
Ethiraj AS, Uttam P, Varunkumar K, Chong KF, Ali GAM (2019) Photocatalytic performance of a novel semiconductor nanocatalyst: copper doped nickel oxide for phenol degradation. Mater Chem Phys 242:122520
Mohapatra S, Rout SR, Das RK, Nayak S, Ghosh SK (2016) Highly hydrophilic luminescent magnetic mesoporous carbon nanospheres for controlled release of anticancer drug and multimodal imaging. Langmuir 32(6):1611–1620
Yue Q, Zhang Y, Wang C, Wang X, Sun Z, Hou X-F, Zhao D, Deng Y (2015) Magnetic yolk–shell mesoporous silica microspheres with supported Au nanoparticles as recyclable high-performance nanocatalysts. J Mater Chem A 3(8):4586–4594
Cui X, Zheng Y, Tian M, Dong Z (2017) Palladium nanoparticles supported on SiO2@Fe3O4@m-MnO2 mesoporous microspheres as a highly efficient and recyclable catalyst for hydrodechlorination of 2, 4-dichlorophenol and reduction of nitroaromatic compounds and organic dyes. Mol Catal 433:202–211
Giahi M, Pathania D, Agarwal S, Ali GAM, Chong KF, Gupta VK (2019) Preparation of Mg-doped TiO2 nanoparticles for photocatalytic degradation of some organic pollutants. Stud Univ Babes-Bolyai Chem 64(1):7–18
Ali GAM, Wahba OAG, Hassan AM, Fouad OA, Chong KF (2015) Calcium-based nanosized mixed metal oxides for supercapacitor application. Ceram Int 41(6):8230–8234
Zhang X, Xie Y, Lin X, Li H, Cao H (2013) An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J Mater Cycles Waste Manage 15(4):420–430
Zeng X, Li J, Singh N (2014) Recycling of spent lithium-ion battery: a critical review. Crit Rev Env Sci Technol 44(10):1129–1165
Ordoñez J, Gago EJ, Girard A (2016) Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew Sustain Energy Rev 60:195–205
Wang W-Y, Yen CH, Lin J-L, Xu R-B (2019) Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J Mater Cycles Waste Manage 21(2):300–307
Shah K, Gupta K, Sengupta B (2017) Selective separation of copper and zinc from spent chloride brass pickle liquors using solvent extraction and metal recovery by precipitation-stripping. J Env Chem Eng 5(5):5260–5269
Cunha ML, Gahan CS, Menad N, Sandström Å (2008) Possibilities to use oxidic by-products for precipitation of Fe/As from leaching solutions for subsequent base metal recovery. Miner Eng 21(1):38–47
Kanamori T, Matsuda M, Miyake M (2009) Recovery of rare metal compounds from nickel–metal hydride battery waste and their application to CH4 dry reforming catalyst. J Hazard Mater 169(1–3):240–245
Aboelazm EAA, Ali GAM, Chong KF (2018) Cobalt oxide supercapacitor electrode recovered from spent lithium-ion battery. Chem Adv Mater 3:67–74
Aboelazm EAA, Ali GAM, Algarni H, Yin H, Zhong YL, Chong KF (2018) Magnetic electrodeposition of the hierarchical cobalt oxide nanostructure from spent lithium-ion batteries: its application as a supercapacitor electrode. J Phys Chem C 122(23):12200–12206
Astruc D (2008) Transition-metal nanoparticles in catalysis: from historical background to the state-of-the art. Nanopart Catal 16:1–48
Somorjai GA, Frei H, Park JY (2009) Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J Am Chem Soc 131(46):16589–16605
Lu AH, EeL S, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46(8):1222–1244
Polshettiwar V, Varma RS (2010) Green chemistry by nano-catalysis. Green Chem 12(5):743–754
Shylesh S, Schuenemann V, Thiel WR (2010) Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 49(20):3428–3459
Claesson EM, Mehendale NC, Gebbink RJK, van Koten G, Philipse AP (2007) Magnetic silica colloids for catalysis. J Magn Magn Mater 311(1):41–45
Yan J-M, Zhang X-B, Akita T, Haruta M, Xu Q (2010) One-step seeding growth of magnetically recyclable Au@ Co core–shell nanoparticles: highly efficient catalyst for hydrolytic dehydrogenation of ammonia borane. J Am Chem Soc 132(15):5326–5327
Torki M, Tangestaninejad S, Mirkhani V, Moghadam M, Mohammadpoor-Baltork I (2014) RuIII (OTf) Salophen CH2–NHSiO2–Fe: an efficient and magnetically recoverable catalyst for trimethylsilylation of alcohols and phenols with hexamethyldisilazane. Appl Organomet Chem 28(4):304–309
Prasad R (2000) Petroleum refining technology, Khanna
Gupta A, Severin D (1997) Characterization of petroleum waxes by high temperature gas chromatography-correlation with physical properties. Pet Sci Technol 15(9–10):943–957
Bennett H (1975) Industrial waxes. Chemical Pub. Co., New York
Letcher C (1984) Waxes. In: Encyclopedia of chemical technology, pp 466–481
Avilino S Jr (1994) Lubricant base oil and wax processing. Morcel Dekker Inc., New York, pp 17–36
Guthrie VB, Guthrie VB (1960) Petroleum products handbook, vol 33. McGraw-Hill, New York.
Concawe PW (1999) Related products. Report (99/110). Concawe, Brussels, p 1
Gottshall R, McCue C (1973) Criteria for quality of petroleum products. Applied Science Publishers Ltd., London (On behalf of the ….)
Freund M (1982) Paraffin products: properties, technologies, applications
Nakagawa H, Tsuge S, Itho T, Kimoto M (1983) Characterization of hydrocarbon waxes by gas–liquid chromatography with a high-resolution glass capillary column. J Chromatogr A 260:391–409
Vainshtein B, Pinsker Z (1950) Opredelenie polozheniya vodoroda v kristallicheskoi reshetke parafina. Dokl Akad Nauk SSSR 72(1):53–56
Meyer G (2009) Thermal properties of micro-crystalline waxes in dependence on the degree of deoiling. SOFW J 135(8):43–50
Yan X, Tian L, He M, Chen X (2015) Three-dimensional crystalline/amorphous Co/Co3O4 core/shell nanosheets as efficient electrocatalysts for the hydrogen evolution reaction. Nano Lett 15(9):6015–6021
Wang Y, Guo J, Wang T, Shao J, Wang D, Yang Y-W (2015) Mesoporous transition metal oxides for supercapacitors. Nanomaterials 5(4):1667–1689
Wang X, Hu A, Meng C, Wu C, Yang S, Hong X (2020) Recent advance in Co3O4 and Co3O4-containing electrode materials for high-performance supercapacitors. Molecules 25(2):269
Yang Q, Lu Z, Sun X, Liu J (2013) Ultrathin Co3O4 nanosheet arrays with high supercapacitive performance. Scientific Reports 3:3537
Pal M, Rakshit R, Singh AK, Mandal K (2016) Ultra high supercapacitance of ultra small Co3O4 nanocubes. Energy 103:481–486
Cheng M, Duan S, Fan H, Su X, Cui Y, Wang R (2017) Core@shell CoO@Co3O4 nanocrystals assembling mesoporous microspheres for high performance asymmetric supercapacitors. Chem Eng J 327:100–108
Liu Z, Zhou W, Wang S, Du W, Zhang H, Ding C, Du Y, Zhu L (2019) Facile synthesis of homogeneous core-shell Co3O4 mesoporous nanospheres as high performance electrode materials for supercapacitor. J Alloy Compd 774:137–144
Zhang F, Yuan C, Lu X, Zhang L, Che Q, Zhang X (2012) Facile growth of mesoporous Co3O4 nanowire arrays on Ni foam for high performance electrochemical capacitors. J Power Sources 203:250–256
Li J, Zhang P, Zhao X, Chen L, Shen J, Li M, Ji B, Song L, Wu Y, Liu D (2019) Structure-controlled Co–Al layered double hydroxides/reduced graphene oxide nanomaterials based on solid-phase exfoliation technique for supercapacitors. J Colloid Interface Sci 549:236–245
Wang P, Zhou H, Meng C, Wang Z, Akhtar K, Yuan A (2019) Cyanometallic framework-derived hierarchical Co3O4–NiO/graphene foam as high-performance binder-free electrodes for supercapacitors. Chem Eng J 369:57–63
Babu IM, William JJ, Muralidharan G (2019) Ordered mesoporous Co3O4/CMC nanoflakes for superior cyclic life and ultra high energy density supercapacitor. Appl Surf Sci 480:371–383
Fan H, Quan L, Yuan M, Zhu S, Wang K, Zhong Y, Chang L, Shao H, Wang J, Zhang J, Cao C-n (2016) Thin Co3O4 nanosheet array on 3D porous graphene/nickel foam as a binder-free electrode for high-performance supercapacitors. Electrochim Acta 188:222–229
Chen H, Wang J, Liao F, Han X, Zhang Y, Xu C, Gao L (2019) Uniform and porous Mn-doped Co3O4 microspheres: Solvothermal synthesis and their superior supercapacitor performances. Ceram Int 45(9):11876–11882
Wang Y, Huo J (2020) C, N co-doped Co3O4 hollow spheres prepared via hydrothermal reaction for improved electrochemical activity. Int J Hydrogen Energy 45(1):1170–1176
Deng S, Xiao X, Chen G, Wang L, Wang Y (2016) Cd doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochim Acta 196:316–327
Dlamini N, Mukaya HE, Van Zyl RL, Chen CT, Zeevaart RJ, Mbianda XY (2019) Synthesis, characterization, kinetic drug release and anticancer activity of bisphosphonates multi-walled carbon nanotube conjugates. Mater Sci Eng C 104:109967
Zhou Y, Liu Y, Zhou X, Gao Y, Gao C, Wang L (2019) High performance p-type organic thermoelectric materials based on metalloporphyrin/single-walled carbon nanotube composite films. J Power Sources 423:152–158
Li G, Chen M, Ouyang Y, Yao D, Lu L, Wang L, Xia X, Lei W, Chen S-M, Mandler D (2019) Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl Surf Sci 469:941–950
Tao F, Zhao Y-Q, Zhang G-Q, Li H-L (2007) Electrochemical characterization on cobalt sulfide for electrochemical supercapacitors. Electrochem Commun 9(6):1282–1287
Yu Z, Tetard L, Zhai L, Thomas J (2015) Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions. Energy Environ Sci 8(3):702–730
Qin Q, Chen L, Wei T, Liu X (2019) MoS2/NiS yolk–shell microsphere-based electrodes for overall water splitting and asymmetric supercapacitor. Small 15(29):1803639
Wang CX, Sun CY, Yu CJ, Li YX, Zhao J, Huang DX, Song YB (2012) Fabrication of highly ordered carbon networks as catalyst supports for aerobic oxidation of glucose. Adv Mater Res 476–478:1186–1192
Zhang F, Gu D, Yu T, Zhang F, Xie S, Zhang L, Deng Y, Wan Y, Tu B, Zhao D (2007) Mesoporous carbon single-crystals from organic−organic self-assembly. J Am Chem Soc 129(25):7746–7747
Shinde SK, Ramesh S, Bathula C, Ghodake GS, Kim DY, Jagadale AD, Kadam AA, Waghmode DP, Sreekanth TVM, Kim HS, Nagajyothi PC, Yadav HM (2019) Novel approach to synthesize NiCo2S4 composite for high-performance supercapacitor application with different molar ratio of Ni and Co. Sci Rep 9(1):13717
Zhu T, Wang Z, Ding S, Chen JS, Lou XWD (2011) Hierarchical nickel sulfide hollow spheres for high performance supercapacitors. RSC Adv 1(3):397–400
Gaikar P, Pawar SP, Mane RS, Nuashad M, Shinde D (2016) Synthesis of nickel sulfide as a promising electrode material for pseudocapacitor application. RSC Adv 6(113):112589–112593
Hsu Y-K, Chen Y-C, Lin Y-G (2014) Synthesis of copper sulfide nanowire arrays for high-performance supercapacitors. Electrochim Acta 139(Suppl C):401–407
Zhang H, Yu X, Guo D, Qu B, Zhang M, Li Q, Wang T (2013) Synthesis of bacteria promoted reduced graphene oxide-nickel sulfide networks for advanced supercapacitors. ACS Appl Mater Interfaces 5(15):7335–7340
Hegde G, Abdul Manaf SA, Kumar A, Ali GAM, Chong KF, Ngaini Z, Sharma KV (2015) Biowaste sago bark based catalyst free carbon nanospheres: waste to wealth approach. ACS Sustain Chem Eng 5(9):2247–2253
Ali GAM, Supriya S, Chong KF, Shaaban ER, Algarni H, Maiyalagan T, Hegde G (2019) Superior supercapacitance behavior of oxygen self-doped carbon nanospheres: a conversion of Allium CEPA peel to energy storage system. Biomass Conv Bioref. https://doi.org/10.1007/s13399-019-00520-3
Ali GAM, Divyashree A, Supriya S, Chong KF, Ethiraj AS, Reddy MV, Algarni H, Hegde G (2017) Carbon nanospheres derived from Lablab purpureus for high performance supercapacitor electrodes: a green approach. Dalton Trans 46(40):14034–14044
Ali GAM, Manaf SAA, Divyashree A, Chong KF, Hegde G (2016) Superior supercapacitive performance in porous nanocarbons. J Energy Chem 25(4):734–739
Ali GAM, Habeeb OA, Algarni H, Chong KF (2018) CaO impregnated highly porous honeycomb activated carbon from agriculture waste: symmetrical supercapacitor study. J Mater Sci 54:683–692
Ali GAM, Abdul Manaf SA, Kumar A, Chong KF, Hegde G (2014) High performance supercapacitor using catalysis free porous carbon nanoparticles. J Phys D Appl Phys 47(49):495307–495313
Zhang R, Lu C, Shi Z, Liu T, Zhai T, Zhou W (2019) Hexagonal phase NiS octahedrons co-modified by 0D-, 1D-, and 2D carbon materials for high-performance supercapacitor. Electrochim Acta 311:83–91
Chen W, Xia C, Alshareef HN (2014) One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 8(9):9531–9541
Wang J, Dong S, Ding B, Wang Y, Hao X, Dou H, Xia Y, Zhang X (2017) Pseudocapacitive materials for electrochemical capacitors: from rational synthesis to capacitance optimization. Nat Sci Rev 4(1):71–90
Liu TC, Pell W, Conway B, Roberson S (1998) Behavior of molybdenum nitrides as materials for electrochemical capacitors comparison with ruthenium oxide. J Electrochem Soc 145(6):1882–1888
Choi D, Blomgren GE, Kumta PN (2006) Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors. Adv Mater 18(9):1178–1182
Balogun M-S, Qiu W, Wang W, Fang P, Lu X, Tong Y (2015) Recent advances in metal nitrides as high-performance electrode materials for energy storage devices. J Mater Chem A 3(4):1364–1387
Li L, Ge J, Wu F, Chen R, Chen S, Wu B (2010) Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J Hazard Mater 176(1):288–293
Saha S, Jana M, Khanra P, Samanta P, Koo H, Murmu NC, Kuila T (2015) Band gap engineering of boron nitride by graphene and its application as positive electrode material in asymmetric supercapacitor device. ACS Appl Mater Interfaces 7(26):14211–14222
Wang Y, Yang W, Yang J (2007) A Co–Al layered double hydroxides nanosheets thin-film electrode fabrication and electrochemical study. Electrochem Solid-State Lett 10(10):A233–A236
Prevot V, Forano C, Khenifi A, Ballarin B, Scavetta E, Mousty C (2011) A templated electrosynthesis of macroporous NiAl layered double hydroxides thin films. Chem Commun 47(6):1761–1763
Gao X, Liu X, Wu D, Qian B, Kou Z, Pan Z, Pang Y, Miao L, Wang J (2019) Significant role of Al in ternary layered double hydroxides for enhancing electrochemical performance of flexible asymmetric supercapacitor. Adv Func Mater 29(36):1903879
Gao X, Zhao Y, Dai K, Wang J, Zhang B, Shen X (2020) NiCoP nanowire@ NiCo-layered double hydroxides nanosheet heterostructure for flexible asymmetric supercapacitors. Chem Eng J 384:123373
Chen H, Hu L, Chen M, Yan Y, Wu L (2014) Nickel-Cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials. Adv Func Mater 24(7):934–942
Guo XL, Liu XY, Hao XD, Zhu SJ, Dong F, Wen ZQ, Zhang YX (2016) Nickel-Manganese layered double hydroxide nanosheets supported on nickel foam for high-performance supercapacitor electrode materials. Electrochim Acta 194(Suppl C):179–186
Shao M, Ning F, Zhao Y, Zhao J, Wei M, Evans DG, Duan X (2012) Core–shell layered double hydroxide microspheres with tunable interior architecture for supercapacitors. Chem Mater 24(6):1192–1197
Zhang L, Wang J, Zhu J, Zhang X, San Hui K, Hui KN (2013) 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials. J Mater Chem A 1(32):9046–9053
Habeeb OA, Ramesh K, Ali GAM, Yunus RM (2017) Low-cost and eco-friendly activated carbon from modified palm kernel shell for hydrogen sulfide removal from wastewater: adsorption and kinetic studies. Desalin Water Treat 84:205–214
Habeeb OA, Ramesh K, Ali GAM, Yunus RM (2017) Experimental design technique on removal of hydrogen sulfide using CaO-eggshells dispersed onto palm kernel shell activated carbon: experiment, optimization, equilibrium and kinetic studies. J Wuhan Univ Technol Mater Sci Ed 32(2):305–320
Habeeb OA, Ramesh K, Ali GAM, Yunus RM (2017) Application of response surface methodology for optimization of palm kernel shell activated carbon preparation factors for removal of H2S from industrial wastewater. J Teknol 79(7):1–10
Habeeb OA, Ramesh K, Ali GAM, Yunus RM (2017) Isothermal modelling based experimental study of dissolved hydrogen sulfide adsorption from waste water using eggshell based activated carbon. Malays J Anal Sci 21(2):334–345
Marina PE, Ali GAM, See LM, Teo EYL, Ng E-P, Chong KF (2016) In situ growth of redox-active iron-centered nanoparticles on graphene sheets for specific capacitance enhancement. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.02.006
Ali GAM, Yusoff MM, Chong KF (2016) Graphene electrochemical production and its energy storage properties. ARPN J Eng Appl Sci 11(16):9712–9717
Gupta VK, Agarwal S, Sadegh H, Ali GAM, Bharti AK, Hamdy AS (2017) Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J Mol Liq 237:466–472
Ali GAM, Thalji MR, Soh WC, Algarni H, Chong KF (2020) One-step electrochemical synthesis of MoS2/graphene composite for supercapacitor application. J Solid State Electrochem 24(1):25–34
Barhoum A, Shalan AE, El-Hout SI, Ali GAM, Abdelbasir SM, Abu Serea ES, Ibrahim AH, Pal K (2019) A broad family of carbon nanomaterials: classification, properties, synthesis, and emerging applications. In: Barhoum A, Bechelany M, Makhlouf A (eds) Handbook of nanofibers. Springer International Publishing, Cham, pp 1–40
Dąbrowski A (2001) Adsorption—from theory to practice. Adv Coll Interface Sci 93(1–3):135–224
Lee SP, Ali GAM, Algarni H, Chong KF (2019) Flake size-dependent adsorption of graphene oxide aerogel. J Mol Liq 277:175–180
DiGiulio D, Wilkin R, Miller C, Oberley G (2011) Investigation of groundwater contamination near Pavillion, Wyoming. US Environmental Protection Agency Draft Report. US EPA. https://www.epa.gov/region8/superfund/wy/pavillion/EPA_ReportOnPavillion_Dec-8-2011.pdf
Machida M, Mochimaru T, Tatsumoto H (2006) Lead (II) adsorption onto the graphene layer of carbonaceous materials in aqueous solution. Carbon 44(13):2681–2688
Li Y-H, Wang S, Luan Z, Ding J, Xu C, Wu D (2003) Adsorption of cadmium (II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 41(5):1057–1062
Zhao H, Baker GA, Holmes SJO (2011) New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Org Biomol Chem 9(6):1908–1916
Ren X, Chen C, Nagatsu M, Wang X (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170(2–3):395–410
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. TrAC Trends Anal Chem 51:33–43
Wu W, Yang Y, Zhou H, Ye T, Huang Z, Liu R, Kuang Y (2013) Highly efficient removal of Cu (II) from aqueous solution by using graphene oxide. Water Air Soil Pollut 224(1):1372
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R (2013) Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans 42(16):5682–5689
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huang Y, Wang X (2011) Removal of Pb (II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans 40(41):10945–10952
Huang Z-H, Zheng X, Lv W, Wang M, Yang Q-H, Kang F (2011) Adsorption of lead (II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27(12):7558–7562
El-Maghrabi HH, Abdelmaged SM, Nada AA, Zahran F, El-Wahab SA, Yahea D, Hussein G, Atrees M (2017) Magnetic graphene based nanocomposite for uranium scavenging. J Hazard Mater 322:370–379
Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, Wang X, Lin N, Qi Y (2013) Adsorption characteristics and behaviors of graphene oxide for Zn (II) removal from aqueous solution. Appl Surf Sci 279:432–440
Zhao J, Wang Z, White JC, Xing B (2014) Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation. Environ Sci Technol 48(17):9995–10009
Yao Y, Yang Z, Zhang D, Peng W, Sun H, Wang S (2012) Magnetic CoFe2O4–graphene hybrids: facile synthesis, characterization, and catalytic properties. Ind Eng Chem Res 51(17):6044–6051
Gollavelli G, Chang C-C, Ling Y-C (2013) Facile synthesis of smart magnetic graphene for safe drinking water: heavy metal removal and disinfection control. ACS Sustain Chem Eng 1(5):462–472
Zhu J, Wei S, Gu H, Rapole SB, Wang Q, Luo Z, Haldolaarachchige N, Young DP, Guo Z (2011) One-pot synthesis of magnetic graphene nanocomposites decorated with core@ double-shell nanoparticles for fast chromium removal. Environ Sci Technol 46(2):977–985
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl Mater Interfaces 4(9):4991–5000
Chandra V, Park J, Chun Y, Lee J, Hwang I, Kim K (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4(7):3979–3986
Liu M, Chen C, Hu J, Wu X, Wang X (2011) Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J Phys Chem C 115(51):25234–25240
WooáLee J, BináKim S (2011) Enhanced Cr (VI) removal using iron nanoparticle decorated graphene. Nanoscale 3(9):3583–3585
Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, Gorring M, Kasner ML, Hou S (2012) Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl Mater Interfaces 4(3):1186–1193
Liu L, Li C, Bao C, Jia Q, Xiao P, Liu X, Zhang Q (2012) Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au (III) and Pd (II). Talanta 93:350–357
Yang Y, Xie Y, Pang L, Li M, Song X, Wen J, Zhao H (2013) Preparation of reduced graphene oxide/poly (acrylamide) nanocomposite and its adsorption of Pb (II) and methylene blue. Langmuir 29(34):10727–10736
Wang L, Jiang L, Su D, Sun C, Chen M, Goh K, Chen Y (2014) Non-covalent synthesis of thermo-responsive graphene oxide–perylene bisimides-containing poly (N-isopropylacrylamide) hybrid for organic pigment removal. J Colloid Interface Sci 430:121–128
Vasudevan S, Lakshmi J (2012) The adsorption of phosphate by graphene from aqueous solution. RSC Adv 2(12):5234–5242
Zhang S, Shao Y, Liu J, Aksay IA, Lin Y (2011) Graphene–polypyrrole nanocomposite as a highly efficient and low cost electrically switched ion exchanger for removing ClO4– from wastewater. ACS Appl Mater Interfaces 3(9):3633–3637
Shi G, Ding Y, Fang H (2012) Unexpectedly strong anion-π interactions on the graphene flakes. J Comput Chem 33(14):1328–1337
Perreault F, Fonseca de Faria A, Elimelech M (2015) Environmental applications of graphene-based nanomaterials. Chem Soc Rev 44(16):5861–5896
Chong MN, Jin B, Chow CW, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44(10):2997–3027
Ravelli D, Dondi D, Fagnoni M, Albini A (2009) Photocatalysis. A multi-faceted concept for green chemistry. Chem Soc Rev 38(7):1999–2011
Xiang Q, Yu J, Jaroniec M (2012) Graphene-based semiconductor photocatalysts. Chem Soc Rev 41(2):782–796
Leary R, Westwood A (2011) Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 49(3):741–772
Khan Z, Chetia TR, Vardhaman AK, Barpuzary D, Sastri CV, Qureshi M (2012) Visible light assisted photocatalytic hydrogen generation and organic dye degradation by CdS–metal oxide hybrids in presence of graphene oxide. RSC Adv 2(32):12122–12128
Li B, Cao H (2011) ZnO@graphene composite with enhanced performance for the removal of dye from water. J Mater Chem 21(10):3346–3349
Wang Y, Wang W, Mao H, Lu Y, Lu J, Huang J, Ye Z, Lu B (2014) Electrostatic self-assembly of BiVO4–reduced graphene oxide nanocomposites for highly efficient visible light photocatalytic activities. ACS Appl Mater Interfaces 6(15):12698–12706
Liu J, Bai H, Wang Y, Liu Z, Zhang X, Sun DD (2010) Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv Func Mater 20(23):4175–4181
Zhang Y, Tang Z-R, Fu X, Xu Y-J (2011) Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: what advantage does graphene have over its forebear carbon nanotube? ACS Nano 5(9):7426–7435
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828
Lambert TN, Chavez CA, Hernandez-Sanchez B, Lu P, Bell NS, Ambrosini A, Friedman T, Boyle TJ, Wheeler DR, Huber DL (2009) Synthesis and characterization of titania−graphene nanocomposites. J Phys Chem C 113(46):19812–19823
Liang Y, Wang H, Casalongue HS, Chen Z, Dai H (2010) TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res 3(10):701–705
Liu X, Pan L, Lv T, Lu T, Zhu G, Sun Z, Sun C (2011) Microwave-assisted synthesis of ZnO–graphene composite for photocatalytic reduction of Cr (VI). Catal Sci Technol 1(7):1189–1193
Williams G, Seger B, Kamat PV (2008) TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2(7):1487–1491
Perera S, Mariano R, Vu K, Nour N, Seitz O, Chabal Y, Balkus K Jr (2012) Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal 2:949–956
Zhu M, Chen P, Liu M (2011) Graphene oxide enwrapped Ag/AgX (X=Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano 5(6):4529–4536
Chen Z, Liu S, Yang M-Q, Xu Y-J (2013) Synthesis of uniform CdS nanospheres/graphene hybrid nanocomposites and their application as visible light photocatalyst for selective reduction of nitro organics in water. ACS Appl Mater Interfaces 5(10):4309–4319
Xiong Z, Zhang LL, Ma J, Zhao X (2010) Photocatalytic degradation of dyes over graphene–gold nanocomposites under visible light irradiation. Chem Commun 46(33):6099–6101
Liu S, Chen Z, Zhang N, Tang Z-R, Xu Y-J (2013) An efficient self-assembly of CdS nanowires–reduced graphene oxide nanocomposites for selective reduction of nitro organics under visible light irradiation. J Phys Chem C 117(16):8251–8261
Zhai C, Zhu M, Bin D, Wang H, Du Y, Wang C, Yang P (2014) Visible-light-assisted electrocatalytic oxidation of methanol using reduced graphene oxide modified Pt nanoflowers-TiO2 nanotube arrays. ACS Appl Mater Interfaces 6(20):17753–17761
Bhunia SK, Jana NR (2014) Reduced graphene oxide-silver nanoparticle composite as visible light photocatalyst for degradation of colorless endocrine disruptors. ACS Appl Mater Interfaces 6(22):20085–20092
El-Maghrabi HH, Nada EA, Soliman FS, Moustafa YM, Amin AE-S (2016) One pot environmental friendly nanocomposite synthesis of novel TiO2-nanotubes on graphene sheets as effective photocatalyst. Egypt J Petrol 25(4):575–584
CDI (2016) Cobalt facts. Cited 15 July 2017. Available from: https://www.cobaltinstitute.org/
Statista (2016) World cobalt reserves as of 2016. Cited 13 Aug 2017. Available from: https://www.statista.com/statistics/264930/global-cobalt-reserves/
Cheang CY, Mohamed N (2016) Removal of cobalt from ammonium chloride solutions using a batch cell through an electrogenerative process. Sep Purif Technol 162:154–161
Pazik PM, Chmielewski T, Glass HJ, Kowalczuk PB (2016) World production and possible recovery of cobalt from the Kupferschiefer stratiform copper ore. E S Web Conf 8:01063
Crundwell FK (2011) Extractive metallurgy of nickel, cobalt and platinum group metals. Elsevier
Nelson E (2017) The hottest thing in the markets right now is an obscure metal mined in DR Congo. Batteries. Cited 15 Aug 2017. Available from: https://qz.com/969115/cobalt-is-the-unlikely-hero-of-financial-markets-right-now/
Randall T (2017) The electric-car boom is so real even oil companies say it’s coming. Available from: https://www.bloomberg.com/news/articles/2017-04-25/electric-car-boom-seen-triggering-peak-oil-demand-in-2030s
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264
Times F (2017) Glencore deals mining blow to African copper producers. Cited 12 Sept 2017. Available from: https://www.ft.com/content/3f1d9664-562f-11e5-a28b-50226830d644
Raugei M, Winfield P (2019) Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J Clean Product 213:926–932
van den Brink S, Kleijn R, Sprecher B, Tukker A (2020) Identifying supply risks by mapping the cobalt supply chain. Resour Conserv Recycl 156:104743
Nada AA, Tantawy HR, Elsayed MA, Bechelany M (2018) Elaboration of nano titania-magnetic reduced graphene oxide for degradation of tartrazine dye in aqueous solution. Solid State Sci 78:116–125
El-Maghrabi HH, Nada AA, Diab KR, Youssef AM, Hamdy A, Roualdesb S, Abd El-Wahab S (2018) Facile fabrication of NiTiO3/graphene nanocomposites for photocatalytic hydrogen generation. J Photochem Photobio A: Chem 365:86–93
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nada, A.A. et al. (2021). Conversion of Waste Cheap Petroleum Paraffinic Wax By-Products to Expensive Valuable Multiple Carbon Nanomaterials. In: Makhlouf, A.S.H., Ali, G.A.M. (eds) Waste Recycling Technologies for Nanomaterials Manufacturing. Topics in Mining, Metallurgy and Materials Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-68031-2_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-68031-2_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68030-5
Online ISBN: 978-3-030-68031-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)