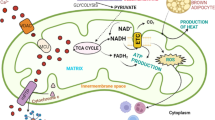
Abstract
Until recently restricted to hereditary mitochondrial diseases, mitochondrial dysfunction is now recognized as a key player and strategic factor in the pathophysiological of many human diseases, ranging from the metabolism, vascular, cardiac, and neurodegenerative diseases to cancer. Because of their participation in a myriad of cellular functions and signaling pathways, precisely identifying the cause of mitochondrial “dysfunctions” can be challenging and requires robust and controlled techniques. Initially limited to the analysis of the respiratory chain functioning, these analytical techniques now enlarge to the analyses of mitochondrial and cellular metabolism, based on metabolomic approaches.
Here, we address the methods used to assay mitochondrial dysfunction, with a highlight on the techniques used in diagnosis on tissues and cells derived from patients, the information they provide, and their strength and weakness.
Targeting mitochondrial dysfunction by various strategies is a huge challenge, requires robust methods of evaluation, and should be able to take into consideration the mitochondria dynamics and localization. The future of mitochondrial medicine is strongly related to a perfect comprehension of its dysfunction.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Burki F (2016) Mitochondrial evolution: going, going, gone. Curr Biol 26:R410–R412. https://doi.org/10.1016/j.cub.2016.04.032
Smith RL, Soeters MR, Wüst RCI, Houtkooper RH (2018) Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev 39:489–517. https://doi.org/10.1210/er.2017-00211
Veloso CD, Belew GD, Ferreira LL et al (2019) A mitochondrial approach to cardiovascular risk and disease. Curr Pharm Des 25:3175–3194. https://doi.org/10.2174/1389203720666190830163735
Onyango IG (2017) Mitochondria in the pathophysiology of Alzheimer’s and Parkinson’s diseases. Front Biosci 22:4521. https://doi.org/10.2741/4521
Jodeiri Farshbaf M, Ghaedi K (2017) Huntington’s disease and mitochondria. Neurotox Res 32:518–529. https://doi.org/10.1007/s12640-017-9766-1
Edeas M, Saleh J, Peyssonnaux C (2020) Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis 97:303–305. https://doi.org/10.1016/j.ijid.2020.05.110
Rongvaux A (2018) Innate immunity and tolerance toward mitochondria. Mitochondrion 41:14–20. https://doi.org/10.1016/j.mito.2017.10.007
Vyas S, Zaganjor E, Haigis MC (2016) Mitochondria and cancer. Cell 166:555–566. https://doi.org/10.1016/j.cell.2016.07.002
Zhang D, Guo R, Lei L et al (2020) COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv 2020.03.24.20042655. doi:https://doi.org/10.1101/2020.03.24.20042655
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Saleh J, Peyssonnaux C, Singh KK, Edeas M (2020) Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion 54:1–7. https://doi.org/10.1016/j.mito.2020.06.008
Lodigiani C, Iapichino G, Carenzo L et al (2020) Venous and arterial thromboembolic complications in {COVID}-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191:9–14. https://doi.org/10.1016/j.thromres.2020.04.024
Giannis D, Ziogas IA, Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 127:104362. https://doi.org/10.1016/j.jcv.2020.104362
Zhang Y, Xiao M, Zhang S et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. NEJM 38:1–3
Oxley TJ, Mocco J, Majidi S et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382:e60. https://doi.org/10.1056/NEJMc2009787
Chevrollier A, Cassereau J, Ferré M et al (2012) Standardized mitochondrial analysis gives new insights into mitochondrial dynamics and OPA1 function. Int J Biochem Cell Biol 44:980–988. https://doi.org/10.1016/j.biocel.2012.03.006
Santidrian AF, Matsuno-Yagi A, Ritland M et al (2013) Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 123:1068–1081. https://doi.org/10.1172/JCI64264
Cantó C, Menzies KJ, Auwerx J (2015) NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22:31–53. https://doi.org/10.1016/j.cmet.2015.05.023
Ježek P, Hlavatá L (2005) Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 37:2478–2503. https://doi.org/10.1016/j.biocel.2005.05.013
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15. https://doi.org/10.1083/jcb.201102095
Anderson NM, Mucka P, Kern JG, Feng H (2018) The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 9:216–237. https://doi.org/10.1007/s13238-017-0451-1
Martínez-Reyes I, Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11:102. https://doi.org/10.1038/s41467-019-13668-3
Romero-Garcia S, Prado-Garcia H (2019) Mitochondrial calcium: transport and modulation of cellular processes in homeostasis and cancer (Review). Int J Oncol. https://doi.org/10.3892/ijo.2019.4696
Quiles JM, Gustafsson ÅB (2020) Mitochondrial quality control and cellular proteostasis: two sides of the same coin. Front Physiol 11:515. https://doi.org/10.3389/fphys.2020.00515
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis. Annu Rev Genet 43:95–118. https://doi.org/10.1146/annurev-genet-102108-134850
Weissig V (2020) Drug development for the therapy of mitochondrial diseases. Trends Mol Med 26:40–57. https://doi.org/10.1016/j.molmed.2019.09.002
Bioenergetics 3 (Nicholls, D. G., and Ferguson, S. J., Academic Press, London, 2002). Biochemistry 69, 818–819 (2004). doi:https://doi.org/10.1023/B:BIRY.0000040210.06512.a7
Hatefi Y (1985) The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem 54:1015–1069. https://doi.org/10.1146/annurev.bi.54.070185.005055
Divakaruni AS, Brand MD (2011) The regulation and physiology of mitochondrial proton leak. Physiology 26:192–205. https://doi.org/10.1152/physiol.00046.2010
De Stefani D, Rizzuto R, Pozzan T (2016) Enjoy the trip: calcium in mitochondria back and forth. Annu Rev Biochem 85:161–192. https://doi.org/10.1146/annurev-biochem-060614-034216
Hsieh VC, Krane EJ, Morgan PG (2017) Mitochondrial disease and anesthesia. J Inborn Errors Metab Screen 5:232640981770777. https://doi.org/10.1177/2326409817707770
Haschke RH, Fink BR (1975) Lidocaine effects on brain mitochondrial metabolism in vitro. Anesthesiology 42:737–739. https://doi.org/10.1097/00000542-197506000-00018
Gellerich FN, Mayr JA, Reuter S et al (2004) The problem of interlab variation in methods for mitochondrial disease diagnosis: enzymatic measurement of respiratory chain complexes. Mitochondrion 4:427–439. https://doi.org/10.1016/j.mito.2004.07.007
Medja F, Allouche S, Frachon P et al (2009) Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion 9:331–339. https://doi.org/10.1016/j.mito.2009.05.001
Signes A, Fernandez-Vizarra E (2018) Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem 62:255–270. https://doi.org/10.1042/EBC20170098
Cogliati S, Lorenzi I, Rigoni G et al (2018) Regulation of mitochondrial electron transport chain assembly. J Mol Biol 430:4849–4873. https://doi.org/10.1016/j.jmb.2018.09.016
Mimaki M, Wang X, McKenzie M et al (2012) Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta Bioenerg 1817:851–862. https://doi.org/10.1016/j.bbabio.2011.08.010
Desquiret-Dumas V, Leman G, Wetterwald C et al (2019) Warburg-like effect is a hallmark of complex I assembly defects. Biochim Biophys Acta Mol basis Dis 1865:2475–2489. https://doi.org/10.1016/j.bbadis.2019.05.011
Wortmann S, Rodenburg RJT, Huizing M et al (2006) Association of 3-methylglutaconic aciduria with sensori-neural deafness, encephalopathy, and Leigh-like syndrome (MEGDEL association) in four patients with a disorder of the oxidative phosphorylation. Mol Genet Metab 88:47–52. https://doi.org/10.1016/j.ymgme.2006.01.013
Giachin G, Bouverot R, Acajjaoui S et al (2016) Dynamics of human mitochondrial complex I assembly: implications for neurodegenerative diseases. Front Mol Biosci 3:43. https://doi.org/10.3389/fmolb.2016.00043
Miwa S, Jow H, Baty K et al (2014) Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun 5:3837. https://doi.org/10.1038/ncomms4837
Letts JA, Fiedorczuk K, Sazanov LA (2016) The architecture of respiratory supercomplexes. Nature 537:644–648. https://doi.org/10.1038/nature19774
Dudkina NV, Sunderhaus S, Boekema EJ, Braun H-P (2008) The higher level of organization of the oxidative phosphorylation system: mitochondrial supercomplexes. J Bioenerg Biomembr 40:419–424. https://doi.org/10.1007/s10863-008-9167-5
Schägger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199:223–231. https://doi.org/10.1016/0003-2697(91)90094-A
Calvaruso MA, Smeitink J, Nijtmans L (2008) Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods 46:281–287. https://doi.org/10.1016/j.ymeth.2008.09.023
Leary SC (2012) Blue native polyacrylamide gel electrophoresis: a powerful diagnostic tool for the detection of assembly defects in the enzyme complexes of oxidative phosphorylation. Methods Mol Biol 837:195–206. https://doi.org/10.1007/978-1-61779-504-6_13
Assouline Z, Jambou M, Rio M et al (2012) A constant and similar assembly defect of mitochondrial respiratory chain complex I allows rapid identification of {NDUFS}4 mutations in patients with Leigh syndrome. Biochim Biophys Acta Mol Basis Dis 1822:1062–1069. https://doi.org/10.1016/j.bbadis.2012.01.013
Wittig I, Braun H-P, Schägger H (2006) Blue native PAGE. Nat Protoc 1:418–428. https://doi.org/10.1038/nprot.2006.62
Mejia EM, Hatch GM (2016) Mitochondrial phospholipids: role in mitochondrial function. J Bioenerg Biomembr 48:99–112. https://doi.org/10.1007/s10863-015-9601-4
Gnaiger E (2008) Polarographic oxygen sensors, the oxygraph, and high-resolution respirometry to assess mitochondrial function. In: Dykens JA, Will Y (eds) Mitochondrial dysfunction in drug-induced toxicity. Wiley, New York, pp 325–352. https://doi.org/10.1002/9780470372531.ch12
Potter M, Lodge TA, Morten KJ (2018) {CHAPTER} 8: monitoring of extracellular and intracellular O2 on a time-resolved fluorescence plate reader. In: Papkovsky DB, Dmitriev RI (eds) Quenched-phosphorescence detection of molecular oxygen: applications in life sciences; RSC detection science series no. 11. The Royal Society of Chemistry, London, pp 175–192
Simonnet H, Vigneron A, Pouysségur J (2014) Conventional techniques to monitor mitochondrial oxygen consumption. Methods Enzymol 542:151–161. https://doi.org/10.1016/B978-0-12-416618-9.00008-X
Grassian AR, Coloff JL, Brugge JS (2011) Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb Symp Quant Biol 76:313–324. https://doi.org/10.1101/sqb.2011.76.010967
Yépez VA, Kremer LS, Iuso A et al (2018) {OCR}-Stats: robust estimation and statistical testing of mitochondrial respiration activities using Seahorse {XF}. Analyzer 13:e0199938. https://doi.org/10.1371/journal.pone.0199938
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. https://doi.org/10.1042/BJ20110162
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat Protoc 2:287–295. https://doi.org/10.1038/nprot.2006.478
Palmer JW, Tandler B, Hoppel CL (1977) Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252:8731–8739
Picard M, Taivassalo T, Ritchie D et al (2009) Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6:e18317. https://doi.org/10.1371/journal.pone.0018317
Zorzano A, Liesa M, Sebastián D et al (2010) Mitochondrial fusion proteins: dual regulators of morphology and metabolism. Semin Cell Dev Biol 21:566–574. https://doi.org/10.1016/j.semcdb.2010.01.002
Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. Semin Cell Dev Biol 150:1283–1298. https://doi.org/10.1083/jcb.150.6.1283
Wai T, Langer T (2016) Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab 27:105–117. https://doi.org/10.1016/j.tem.2015.12.001
Piper HM, Sezer O, Schleyer M et al (1985) Development of ischemia-induced damage in defined mitochondrial subpopulations. J Mol Cell Cardiol 17:885–896. https://doi.org/10.1016/s0022-2828(85)80102-4
Affourtit C, Brand MD, Al Amir Dache Z et al (2018) Understanding mitochondrial complex I assembly in health and disease. Mitochondrion 9:818–819. https://doi.org/10.1042/EBC20170098
Gueguen N, Lefaucheur L, Ecolan P et al (2005) Ca 2+-activated myosin-ATPases, creatine and adenylate kinases regulate mitochondrial function according to myofibre type in rabbit. J Physiol 564:723–735. https://doi.org/10.1113/jphysiol.2005.083030
Kuznetsov AV, Veksler V, Gellerich FN et al (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3:965–976. https://doi.org/10.1038/nprot.2008.61
Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383–393
Nicholls DG, Bernson VS (1977) Inter-relationships between proton electrochemical gradient, adenine-nucleotide phosphorylation potential and respiration, during substrate-level and oxidative phosphorylation by mitochondria from brown adipose tissue of cold-adapted guinea-pigs. Eur J Biochem 75:601–612. https://doi.org/10.1111/j.1432-1033.1977.tb11560.x
Gnaiger E, Kemp RB (1990) Anaerobic metabolism in aerobic mammalian cells: information from the ratio of calorimetric heat flux and respirometric oxygen flux. Biochim Biophys Acta Bioenerg 1016:328–332. https://doi.org/10.1016/0005-2728(90)90164-Y
Brown GC, Lakin-Thomas PL, Brand MD (1990) Control of respiration and oxidative phosphorylation in isolated rat liver cells. Eur J Biochem 192:355–362. https://doi.org/10.1111/j.1432-1033.1990.tb19234.x
Affourtit C, Brand MD (2009) Chapter 23 measuring mitochondrial bioenergetics in INS-1E insulinoma cell. Methods Enzymol 457:405–424
Kane MS, Paris A, Codron P et al (2018) Current mechanistic insights into the CCCP-induced cell survival response. Biochem Pharmacol 148:100–110. https://doi.org/10.1016/j.bcp.2017.12.018
Stepanova A, Konrad C, Manfredi G et al (2019) The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem 148:731–745. https://doi.org/10.1111/jnc.14654
Yadava N, Nicholls DG (2007) Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27:7310–7317. https://doi.org/10.1523/JNEUROSCI.0212-07.2007
Rodríguez-Enríquez S, Juárez O, Rodríguez-Zavala JS, Moreno-Sánchez R (2001) Multisite control of the Crabtree effect in ascites hepatoma cells. Eur J Biochem 268:2512–2519. https://doi.org/10.1046/j.1432-1327.2001.02140.x
Nicholls DG (2010) Mitochondrial ion circuits. Essays Biochem 47:25–35. https://doi.org/10.1042/bse0470025
Zhang Q, Padayatti PS, Leung JH (2017) Proton-translocating nicotinamide nucleotide transhydrogenase: a structural perspective. Front Physiol 8:1089. https://doi.org/10.3389/fphys.2017.01089
Poburko D, Demaurex N (2012) Regulation of the mitochondrial proton gradient by cytosolic Ca2+ signals. Pflugers Arch 464:19–26. https://doi.org/10.1007/s00424-012-1106-y
Cadenas S (2018) Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg 1859:940–950. https://doi.org/10.1016/j.bbabio.2018.05.019
Mignotte B, Vayssiere JL (1998) Mitochondria and apoptosis. Eur J Biochem 252:1–15. https://doi.org/10.1046/j.1432-1327.1998.2520001.x
Cottet-Rousselle C, Ronot X, Leverve X, Mayol J-F (2011) Cytometric assessment of mitochondria using fluorescent probes. Cytom Part A 79A:405–425. https://doi.org/10.1002/cyto.a.21061
Perry SW, Norman JP, Barbieri J et al (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. BioTechniques 50:98–115. https://doi.org/10.2144/000113610
Ward MW, Rego AC, Frenguelli BG, Nicholls DG (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20:7208–7219
Creed S, McKenzie M (2019) Measurement of mitochondrial membrane potential with the fluorescent dye tetramethylrhodamine methyl ester (TMRM). Methods Mol Biol 1928:69–76
Rahn CA, Bombick DW, Doolittle DJ (1991) Assessment of mitochondrial membrane potential as an indicator of cytotoxicity. Fundam Appl Toxicol 16:435–448. https://doi.org/10.1016/0272-0590(91)90084-h
Moreno AJ, Santos DL, Magalhães-Novais S, Oliveira PJ (2015) Measuring mitochondrial membrane potential with a tetraphenylphosphonium-selective electrode. Curr Protoc Toxicol 65:25.5.1–25.5.16. https://doi.org/10.1002/0471140856.tx2505s65
Renner-Sattler K, Fasching M, Gnaiger E (2016) Mitochondrial Physiology Network 14.05(04):1–13, Technical note. https://wiki.oroboros.at/index.php/MiPNet14.05_TPP-mtMembranePotential
Trijbels JMF, Sengers RCA, Ruitenbeek W et al (1988) Disorders of the mitochondrial respiratory chain: clinical manifestations and diagnostic approach. Eur J Pediatr 148:92–97. https://doi.org/10.1007/BF00445910
Carrozzo R, Dionisi-Vici C, Steuerwald U et al (2007) {SUCLA}2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 130:862–874. https://doi.org/10.1093/brain/awl389
Chao De La Barca JM, Mirebeau-Prunier D, Moal V et al (2015) Metabolome and mass spectrometry: new biomedical analysis perspectives. Ann Biol Clin (Paris) 73:126–130. https://doi.org/10.1684/abc.2014.1020
Esterhuizen K, van der Westhuizen FH, Louw R (2017) Metabolomics of mitochondrial disease. Mitochondrion 35:97–110. https://doi.org/10.1016/j.mito.2017.05.012
Kouassi Nzoughet J, Chao de la Barca JM, Guehlouz K et al (2019) Nicotinamide deficiency in primary open-angle glaucoma. Investig Opthalmol Vis Sci 60:2509. https://doi.org/10.1167/iovs.19-27099
Nikkanen J, Forsström S, Euro L et al (2016) Mitochondrial {DNA} replication defects disturb cellular {dNTP} pools and remodel one-carbon metabolism. Cell Metab 23:635–648. https://doi.org/10.1016/j.cmet.2016.01.019
Khan NA, Nikkanen J, Yatsuga S et al (2017) mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab 26:419–428.e5. https://doi.org/10.1016/j.cmet.2017.07.007
Bocca C, Kane MS, Veyrat-Durebex C et al (2018) The metabolomic bioenergetic signature of opa1-disrupted mouse embryonic fibroblasts highlights aspartate deficiency. Sci Rep 8:11528. https://doi.org/10.1038/s41598-018-29972-9
Rahman J, Rahman S (2018) Mitochondrial medicine in the omics era. Lancet 391:2560–2574. https://doi.org/10.1016/S0140-6736(18)30727-X
Tripodi F, Castoldi A, Nicastro R et al (2018) Methionine supplementation stimulates mitochondrial respiration. Biochim Biophys Acta, Mol Cell Res 1865:1901–1913. https://doi.org/10.1016/j.bbamcr.2018.09.007
Andréasson C, Ott M, Büttner S (2019) Mitochondria orchestrate proteostatic and metabolic stress responses. EMBO Rep 20:e47865. https://doi.org/10.15252/embr.201947865
Yi H-S, Chang JY, Shong M (2018) The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases. J Mol Endocrinol 61:R91–R105. https://doi.org/10.1530/JME-18-0005
Mick E, Titov DV, Skinner OS et al (2020) Distinct mitochondrial defects trigger the integrated stress response depending on the metabolic state of the cell. elife 9:e49178. https://doi.org/10.7554/eLife.49178
Miliotis S, Nicolalde B, Ortega M et al (2019) Forms of extracellular mitochondria and their impact in health. Mitochondrion 48:16–30. https://doi.org/10.1016/j.mito.2019.02.002
Al Amir Dache Z, Otandault A, Tanos R et al (2020) Blood contains circulating cell-free respiratory competent mitochondria. FASEB J 34:3616–3630. https://doi.org/10.1096/fj.201901917RR
Hayakawa K, Chan SJ, Mandeville ET et al (2018) Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells 36:1404–1410. https://doi.org/10.1002/stem.2856
Caicedo A, Aponte PM, Cabrera F et al (2017) Artificial mitochondria transfer: current challenges, advances, and future applications. Stem Cells Int 2017:7610414. https://doi.org/10.1155/2017/7610414
Acknowledgments
The authors thank the following institutions and patient associations: Université d’Angers and CHU d’Angers, Fondation Maladies Rares and UNADEV.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Gueguen, N., Lenaers, G., Reynier, P., Weissig, V., Edeas, M. (2021). Mitochondrial Dysfunction in Mitochondrial Medicine: Current Limitations, Pitfalls, and Tomorrow. In: Weissig, V., Edeas, M. (eds) Mitochondrial Medicine . Methods in Molecular Biology, vol 2276. Springer, New York, NY. https://doi.org/10.1007/978-1-0716-1266-8_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1266-8_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-0716-1265-1
Online ISBN: 978-1-0716-1266-8
eBook Packages: Springer Protocols