Abstract
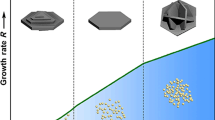
Surface structure control of functional nano-/micro-crystallites has attracted great attention because many important physicochemical properties depend on their surface. Guided by the supersaturation-dependent surface structure evolution strategy we proposed recently, NaTaO3 submicrometer crystals with morphologies of cubes, corner truncated cubes, edge and corner truncated cubes, and quasi-spheres can be synthesized by changing the volume ratio of ethylene glycol to water and the amount of NaOH in the composite solvent. Under low supersaturation condition, NaTaO3 cubic crystals with low energy {100} facets were obtained. As the supersaturation increases, the corners and edges of NaTaO3 cubic crystals, which possess higher surface energy, were gradually truncated. Surprisingly, quasi-sphere crystallites formed under extremely high supersaturation condition, which is difficult to be explained by the classical crystal growth theories. By analyzing the formation work of two-dimension crystal nuclei, we concluded that the crystal growth tend to be isotropic at extremely high supersaturation, which well explained the formation of the quasi-sphere crystallites.
摘要
表面结构决定了晶体材料的许多重要的物理化学性质. 可控制备具有特定表面结构的微纳米晶体, 从而实现对晶体材料性能的改善, 引起了广泛的关注. 本课题组近期相关的研究表明, 晶体生长体系中, 生长单元的过饱和度决定了晶体的表面结构. 基于已有研究结果, 本文通过调节混合溶剂体系中乙二醇和水的相对体积比及NaOH的用量, 改变晶体生长体系中生长单元的过饱和度, 合成了亚微米尺寸的NaTaO3立方体、 削角立方体、 削角削棱立方体和准球体. 低过饱和度条件下, 形成的NaTaO3颗粒是低能{100}晶面裸露的立方体; 随过饱和度的增加, 立方体的角和棱逐渐削去, 裸露表面能更高的晶面; 极高过饱和度条件下, 则形成准球体NaTaO3颗粒. 这种准球体的形成难以用经典的晶体生长理论加以解释. 通过研究同样受过饱和度影响的二维晶核生成功(W hkl ), 发现在极高的过饱和度的条件下, 晶体生长趋于各向同性, 合理地解释了准球体的形成机制.
Similar content being viewed by others
References
Tian N, Zhou ZY, Sun SG, Ding Y, Wang ZL. Synthesis of tetra-hexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science, 2007, 316: 732–735
Yang HG, Sun CH, Qiao SZ, et al. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature, 2008, 453: 638–641
Han X, Kuang Q, Jin M, Xie Z, Zheng L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J Am Chem Soc, 2009, 131: 3152–3153
Han X, Jin M, Xie S, et al. Synthesis of tin dioxide octahedral nanoparticles with exposed high-energy {221} facets and enhanced gas-sensing properties. Angew Chem Int Ed, 2009, 48: 9180–9183
Kuang Q, Wang X, Jiang Z, Xie Z, Zheng L. High-energy-surface engineered metal oxide micro- and nanocrystallites and their applications. Acc Chem Res, 2014, 47: 308–318
Markov IV. Crystal Growth for Beginners: Fundamentals of Nucleation, Crystal Growth and Epitaxy (2nd Edition). Singapore: World Scientific Publishing, 2003
Wang Y, He J, Liu C, Chong WH, Chen H. Thermodynamics versus kinetics in nanosynthesis. Angew Chem Int Ed, 2015, 54: 2022–2051
Lin HX, Lei ZC, Jiang ZY, et al. Supersaturation-dependent surface structure evolution: from ionic, molecular to metallic micro/nanocrystals. J Am Chem Soc, 2013, 135: 9311–9314
Kato H, Kudo A. Highly efficient decomposition of pure water into H2 and O2 over NaTaO3 photocatalysts. Catal Lett, 1999, 58: 153–155
Zhang Y, Chen Y, Zhang YP, et al. A novel humidity sensor based on NaTaO3 nanocrystalline. Sensor Actuat B Chem, 2012, 174: 485–489
Kato H, Kudo A. New tantalate photocatalysts for water decomposition into H2 and O2. Chem Phys Lett, 1998, 295: 487–492
Kato H, Kudo A. Water splitting into H2 and O2 on alkali tantalate photocatalysts ATaO3 (A = Li, Na, and K). J Phys Chem B, 2001, 105: 4285–4292
Kanhere P, Zheng JW, Chen Z. Visible light driven photocatalytic hydrogen evolution and photophysical properties of Bi3+ doped NaTaO3. Int J Hydrogen Energ, 2012, 37: 4889–4896
Kato H, Asakura K, Kudo A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J Am Chem Soc, 2003, 125: 3082–3089
Liu DR, Wei CD, Xue B, Zhang XG, Jiang YS. Synthesis and photocatalytic activity of N-doped NaTaO3 compounds calcined at low temperature. J Hazard Mater, 2010, 182: 50–54
Liu DR, Jiang YS, Gao GM. Photocatalytic degradation of an azo dye using N-doped NaTaO3 synthesized by one-step hydrothermal process. Chemosphere, 2011, 83: 1546–1552
Jiang W, Jiao XL, Chen DR. Photocatalytic water splitting of surfactant-free fabricated high surface area NaTaO3 nanocrystals. Int J Hydrogen Energ, 2013, 38: 12739–12746
Li X, Zang JL. Facile hydrothermal synthesis of sodium tantalate (NaTaO3) nanocubes and high photocatalytic properties. J Phys Chem C, 2009, 113: 19411–19418
Liang S, Shen L, Zhu J, et al. Morphology-controlled synthesis and efficient photocatalytic performances of a new promising photocatalyst Sr0.25H1.5Ta2O6 center dot H2O. RSC Adv, 2011, 1: 458–467
Ouyan JJ, Pei J, Kuang Q, Xie ZX, Zheng LS. Supersaturation-controlled shape evolution of alpha-Fe2O3 nanocrystals and their facet-dependent catalytic and sensing properties. ACS Appl Mater Interfaces, 2014, 6: 12505
He Y, Zhu YF, Wu NZ. Mixed solvents: a key in solvothermal synthesis of KTaO3. J Solid State Chem, 2004, 177: 2985–2990
Makarova M, Bykov P, Drahokoupil J, et al. Solvothermal synthesis of nanocrystalline KTaO3: effect of solvent dielectric constant. Mater Res Bull, 2012, 47: 1768–1773
Sivadas N, Dixit H, Cooper VR, Xiao D. Thickness-dependent carrier density at the surface of SrTiO3 (111) slabs. Phys Rev B, 2014, 89: 070305
Eglitis RI. First-principles calculations of the atomic and electronic structure of CaTiO3 (111) surfaces. Ferroelectrics, 2011, 424: 1–6
Liu W, Wang CC, Cui J, Man ZY. Ab initio calculations of the CaTiO3 (111) polar surfaces. Solid State Commun, 2009, 149: 1871–1876
Eglitis RI, Vanderbilt D. First-principles calculations of atomic and electronic structure of SrTiO3 (001) and (011) surfaces. Phys Rev B, 2008, 77: 195408
Eglitis RI, Vanderbilt D. Ab initio calculations of the atomic and electronic structure of CaTiO3 (001) and (011) surfaces. Phys Rev B, 2008, 78: 155420
Pangarov NA. The crystal orientation of electrodeposited metals. Electrochim Acta, 1962, 7: 139–146
Pangarov NA. On the crystal orientation of electrodeposited metals. Electrochim Acta, 1964, 9: 721–726
Kumar VB, Gedanken A, Kimmel G, Porat Z. Ultrasonic cavitation of molten gallium: formation of micro- and nano-spheres. Ultrason Sonochem, 2014, 21: 1166–1173
Wang YL, Xia YN. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett, 2004, 4: 2047–2050
Wang HQ, Miyauchi M, Ishikawa Y, et al. Single-crystalline rutile TiO2 hollow spheres: room-temperature synthesis, tailored visible-light-extinction, and effective scattering layer for quantum dot-sensitized solar cells. J Am Chem Soc, 2011, 133: 19102–19109
Wang H, Koshizaki N, Li L, et al. Size-tailored ZnO submicrometer spheres: bottom-up construction, size-related optical extinction, and selective aniline trapping. Adv Mater, 2011, 23: 1865–1870
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wei Chen was born in 1984. He received his PhD degree in chemistry from the Department of Chemistry, Fuzhou University, Fuzhou, China, in 2012. Currently, he is a post-doctor at the State Key Laboratory of Physical Chemistry of Solid Surfaces & Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University. His research interests include the control of surface and interface structures and the study of catalytic, photocatalytic and gas-sensing performances of micro- and nanocrystallines.
Qin Kuang was born in 1978. He received his BSc degree in 2001 and PhD degree in 2008 from Xiamen University, China. Thereafter, he joined the Department of Chemistry as an assistant professor in Xiamen University and was promoted to associate professor in 2009. His current research focuses on the surface/interface engineering of inorganic functional nanomaterials and their applications in energy and environmental fields.
Zhaoxiong Xie was born in 1968. He received his BSc degree (1987) in chemistry, MSc degree (1990), and PhD degree (1995) in physical chemistry from Xiamen University, China. He worked as a postdoctoral fellow at Centre d’Etudes de Saclay in France from 1997 to 1998. Since 2002, he has been a professor of physical chemistry at Xiamen University. His current research is focused on the surface/interface chemistry of inorganic nanomaterials.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, W., Kuang, Q. & Xie, Z. Morphology evolution of NaTaO3 submicrometer single-crystals: from cubes to quasi-spheres. Sci. China Mater. 58, 281–288 (2015). https://doi.org/10.1007/s40843-015-0041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-015-0041-6