Abstract
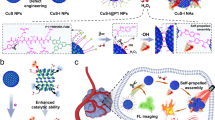
Individual inorganic nanoparticles (NPs) have been widely used in the fields of drug delivery, cancer imaging and therapy. There are still many hurdles that limit the performance of individual NPs for these applications. The utilization of highly ordered NP ensembles opens a door to resolve these problems, as a result of their new or advanced collective properties. The assembled NPs show several advantages over individual NP-based systems, such as improved cell internalization and tumor targeting, enhanced multimodality imaging capability, superior combination therapy arising from synergistic effects, possible complete clearance from the whole body by degradation of assemblies into original small NP building blocks, and so on. In this review, we discuss the potential of utilizing assembled NP ensembles for cancer imaging and treatment by taking plasmonic vesicular assemblies of Au NPs as an example. We first summarize the recent developments in the self-assembly of plasmonic vesicular structures of NPs from amphiphilic polymer-tethered NP building blocks. We further review the utilization of plasmonic vesicles of NPs for cancer imaging (e.g. multi-photon induced luminescence, photothermal, and photoacoustic imaging), and cancer therapy (e.g., photothermal therapy, and chemotherapy). Finally, we outline current challenges and our perspectives along this line.

Similar content being viewed by others
References
Wiedmann, T.; Sadhukha, T.; Hammer, B.; Panyam, J. Image-guided drug delivery in lung cancer. Drug Deliv. Transl. Res. 2012, 2, 31–44.
Mitsudomi, T.; Suda, K.; Yatabe, Y. Surgery for NSCLC in the era of personalized medicine. Nat. Rev. Clin. Oncol. 2013, 10, 235–244.
Devarakonda, S.; Morgensztern, D.; Govindan, R. Molecularly targeted therapies in locally advanced non-small-cell lung cancer. Clin. Lung Cancer 2013, 14, 467–472.
Govindan, R.; Bogart, J.; Vokes, E. E. Locally advanced non-small cell lung cancer: The past, present, and future. J. Thorac. Oncol. 2008, 3, 917–928.
Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284.
Sukumar, U.; Bhushan, B.; Dubey, P.; Matai, I.; Sachdev, A.; Packirisamy, G. Emerging applications of nanoparticles for lung cancer diagnosis and therapy. Int. Nano Lett. 2013, 3, 1–17.
Janib, S. M.; Moses, A. S.; MacKay, J. A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Delivery Rev. 2010, 62, 1052–1063.
Chan, W. C. W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018.
Larson, D. R.; Zipfel, W. R.; Williams, R. M.; Clark, S. W.; Bruchez, M. P.; Wise, F. W.; Webb, W. W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436.
Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544.
Chen, J. Y.; Yang, M. X.; Zhang, Q. A.; Cho, E. C.; Cobley, C. M.; Kim, C.; Glaus, C.; Wang, L. H. V.; Welch, M. J.; Xia, Y. N. Gold nanocages: A novel class of multifunctional nanomaterials for theranostic applications. Adv. Funct. Mater. 2010, 20, 3684–3694.
Kennedy, L. C.; Bickford, L. R.; Lewinski, N. A.; Coughlin, A. J.; Hu, Y.; Day, E. S.; West, J. L.; Drezek, R. A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183.
Dreaden, E. C.; Alkilany, A. M.; Huang, X. H.; Murphy, C. J.; El-Sayed, M. A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779.
Wang, Y. C.; Black, K. C. L.; Luehmann, H.; Li, W. Y.; Zhang, Y.; Cai, X.; Wan, D. H.; Liu, S. Y.; Li, M.; Kim, P.; et al. A. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013, 7, 2068–2077.
Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671.
Nie, Z.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15–25.
Jones, M. R.; Osberg, K. D.; Macfarlane, R. J.; Langille, M. R.; Mirkin, C. A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827.
Glotzer, S. C.; Solomon, M. J. Anisotropy of building blocks and their assembly into complex structures. Nat. Mater. 2007, 6, 557–562.
Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653.
Zhuang, J.; Wu, H.; Yang, Y.; Cao, Y. C. Supercrystalline colloidal particles from artificial atoms. J. Am. Chem. Soc. 2007, 129, 14166–14167.
Wang, L. B.; Xu, L. G.; Kuang, H.; Xu, C. L.; Kotov, N. A. Dynamic nanoparticle assemblies. Acc. Chem. Res. 2012, 45, 1916–1926.
Gong, J.; Li, G.; Tang, Z. Self-assembly of noble metal nanocrystals: Fabrication, optical property, and application. Nano Today 2012, 7, 564–585.
Hao, X.; Shang, X.; Wu, J.; Shan, Y.; Cai, M.; Jiang, J.; Huang, Z.; Tang, Z.; Wang, H. Single-particle tracking of hepatitis B virus-like vesicle entry into cells. Small 2011, 7, 1212–1218.
Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329.
He, J.; Huang, X.; Li, Y.-C.; Liu, Y.; Babu, T.; Aronova, M. A.; Wang, S.; Lu, Z.; Chen, X.; Nie, Z. Self-assembly of amphiphilic plasmonic micelle-like nanoparticles in selective solvents. J. Am. Chem. Soc. 2013, 135, 7974–7984.
He, J.; Liu, Y.; Babu, T.; Wei, Z.; Nie, Z. Self-assembly of inorganic nanoparticle vesicles and tubules driven by tethered linear block copolymers. J. Am. Chem. Soc. 2012, 134, 11342–11345.
He, J.; Wei, Z.; Wang, L.; Tomova, Z.; Babu, T.; Wang, C.; Han, X.; Fourkas, J. T.; Nie, Z. Hydrodynamically driven self-assembly of giant vesicles of metal nanoparticles for remote-controlled release. Angew. Chem. Int. Ed. 2013, 52, 2463–2468.
Chou, L. Y. T.; Zagorovsky, K.; Chan, W. C. W. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nanotechnol. 2014, 9, 148–155.
Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964.
Guo, X.; Szoka, F. C. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc. Chem. Res. 2003, 36, 335–341.
Sawant, R. R.; Torchilin, V. P. Liposomes as “smart” pharmaceutical nanocarriers. Soft Matter 2010, 6, 4026–4044.
Percec, V.; Wilson, D. A.; Leowanawat, P.; Wilson, C. J.; Hughes, A. D.; Kaucher, M. S.; Hammer, D. A.; Levine, D. H.; Lim, A. J.; Bates, F. S.; et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 2010, 328, 1009–1014.
Zhang, X.; Rehm, S.; Safont-Sempere, M. M.; Würthner, F. Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems. Nat. Chem. 2009, 1, 623–629.
Discher, B. M.; Won, Y. Y.; Ege, D. S.; Lee, J. C. M.; Bates, F. S.; Discher, D. E.; Hammer, D. A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146.
Huang, H. Y.; Remsen, E. E.; Kowalewski, T.; Wooley, K. L. Nanocages derived from shell cross-linked micelle templates. J. Am. Chem. Soc. 1999, 121, 3805–3806.
Nie, Z.; Fave, D.; Kumacheva E.; Zou, S.; Walker, G. C.; Rubinstein, M. Self-assembly of metal-polymer analogues of amphiphilic triblock copolymers. Nat. Mater. 2007, 6, 609–614.
Nikolic, M. S.; Olsson, C.; Salcher, A.; Kornowski, A.; Rank, A.; Schubert, R.; Frömsdorf, A.; Weller, H.; Förster, S. Micelle and vesicle formation of amphiphilic nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2752–2754.
Zubarev, E. R.; Xu, J.; Sayyad, A.; Gibson, J. D. Amphiphilicity-driven organization of nanoparticles into discrete assemblies. J. Am. Chem. Soc. 2006, 128, 15098–15099.
Hu, J.; Wu, T.; Zhang, G.; Liu, S. Efficient synthesis of single gold nanoparticle hybrid amphiphilic triblock copolymers and their controlled self-assembly. J. Am. Chem. Soc. 2012, 134, 7624–7627.
Song, J.; Cheng, L.; Liu, A.; Yin, J.; Kuang, M.; Duan, H. Plasmonic vesicles of amphiphilic gold nanocrystals self-assembly and external-stimuli-triggered destruction. J. Am. Chem. Soc. 2011, 133, 10760–10763.
Guo, Y.; Harirchian-Saei, S.; Izumi, C. M. S.; Moffitt, M. G. Block copolymer mimetic self-assembly of inorganic nanoparticles. ACS Nano 2011, 5, 3309–3318.
Wang, B.; Li, B.; Dong, B.; Zhao, B.; Li, C. Y. Homo- and hetero-particle clusters formed by Janus nanoparticles with bicompartment polymer brushes. Macromolecules 2010, 43, 9234–9238.
Andala, D. M.; Shin, S. H. R.; Lee, H.-Y. Bishop, K. J. M. Templated synthesis of amphiphilic nanoparticles at the liquid-liquid interface. ACS Nano 2012, 6, 1044–1050.
Wei, K.; Li, J.; Liu, J.; Chen, G.; Jiang, M. Reversible vesicles of supramolecular hybrid nanoparticles. Soft Matter 2012, 8, 3300–3303.
Liu, Y.; Li, Y.; He, J.; Duelge, K. J.; Lu, Z.; Nie, Z. Entropy-driven pattern formation of hybrid vesicular assemblies made from molecular and nanoparticle amphiphiles. J. Am. Chem. Soc. 2014, 136, 2602–2610.
Song, J.; Zhou, J.; Duan, H. Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J. Am. Chem. Soc. 2012, 134, 13458–13469.
Niikura, K.; Iyo, N.; Higuchi, T.; Nishio, T.; Jinnai, H.; Fujitani, N.; Ijiro, K. Gold nanoparticles coated with semi-fluorinated oligo(ethylene glycol) produce sub-100 nm nanoparticle vesicles without templates. J. Am. Chem. Soc. 2012, 134, 7632–7635.
Song, J.; Pu, L.; Zhou, J.; Duan, B.; Duan, H. Biodegradable theranostic plasmonic vesicles of amphiphilic gold nanorods. ACS Nano 2013, 7, 9947–9960.
Llevot, A.; Astruc, D. Applications of vectorized gold nanoparticles to the diagnosis and therapy of cancer. Chem. Soc. Rev. 2012, 41, 242–257.
Erathodiyil, N.; Ying, J. Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935.
Cheng, Y.; Samia, A. C.; Li, J.; Kenney, M. E.; Resnick, A.; Burda, C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir 2010, 26, 2248–2255.
Ghosh, P.; Han, G.; De, M.; Kim, C. K.; Rotello, V. M. Gold nanoparticles in delivery applications. Adv. Drug Delivery Rev. 2008, 60, 1307–1315.
He, J.; Zhang, P.; Babu, T.; Liu, Y.; Gong, J.; Nie, Z. Near-infrared light-responsive vesicles of Au nanoflowers. Chem. Commun. 2013, 49, 576–578.
Niikura, K.; Iyo, N.; Matsuo, Y.; Mitomo, H.; Ijiro, K. Sub-100 nm gold nanoparticle vesicles as a drug delivery carrier enabling rapid drug release upon light irradiation. ACS Appl. Mater. Inter. 2013, 5, 3900–3907.
Song, J.; Fang, Z.; Wang, C.; Zhou, J.; Duan, B.; Pu, Lu.; Duan, H. Photolabile plasmonic vesicles assembled from amphiphilic gold nanoparticles for remote-controlled traceable drug delivery. Nanoscale 2013, 5, 5816–5824.
Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670.
Gopalakrishnan, G.; Danelon, C.; Izewska, P.; Prummer, M.; Bolinger, P.; Geissbühler, I.; Demurtas, D.; Dubochet, J.; Vogel, H. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem. Int. Ed. 2006, 45, 5478–5483.
Al-Jamal, W. T.; Al-Jamal, K. T.; Tian, B.; Lacerda, L.; Bomans, P. H.; Frederik, P. M.; Kostarelos, K. Lipid-quantum dot bilayer vesicles enhance tumor cell uptake and retention in vitro and in vivo. ACS Nano 2008, 2, 408–418.
Niikura, K.; Iyo, N.; Higuchi, T.; Nishio, T.; Jinnai, H.; Fujitani, N.; Ijiro, K. Gold nanoparticles coated with semi-fluorinated oligo(ethylene glycol) produce sub-100 nm nanoparticle vesicles without templates. J. Am. Chem. Soc. 2012, 134, 7632–7635.
Bian, T.; Shang, L.; Yu, H.; Perez, M. T.; Wu, L.-Z.; Tung, C.-H.; Nie, Z.; Tang, Z.; Zhang, T. Spontaneous organization of inorganic nanoparticles into nanovesicles triggered by UV light. Adv. Mater. 2014, in press, DOI: 10.1002/adma.201401182.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Yin, JJ. & Nie, Z. Harnessing the collective properties of nanoparticle ensembles for cancer theranostics. Nano Res. 7, 1719–1730 (2014). https://doi.org/10.1007/s12274-014-0541-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-014-0541-9