Abstract
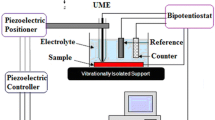
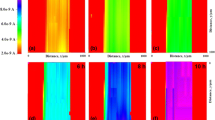
The effect of corrosion protection performance of epoxy coatings containing magnesium (Mg) nanoparticles on carbon steel was analyzed using scanning electrochemical microscopy (SECM) and electrochemical impedance spectroscopy (EIS). Localized measurements such as oxygen consumption and iron dissolution were observed using SECM in 0.1 M NaCl in the epoxy-coated sample. Line profile and topographic image analysis were measured by applying −0.70 and +0.60 V vs the Ag/AgCl/saturated KCl reference electrode as the tip potential for the cathodic and anodic reactions, respectively. The tip current at −0.70 V for the epoxy-coated sample with Mg nanoparticles decreased rapidly, which is due to cathodic reduction in dissolved oxygen. The EIS measurements were conducted in 0.1 M NaCl after wet and dry cyclic corrosion test. The increase in the film resistance (R f) and charge transfer resistance (R ct) values was confirmed by the addition of Mg nanoparticles in the epoxy coating. Scanning electron microscope/energy-dispersive X-ray spectroscope analysis showed that Mg was enriched in corrosion products at a scratched area of the coated steel after corrosion testing. Focused ion beam–transmission electron microscope analysis confirmed the presence of the nanoscale oxide layer of Mg in the rust of the steel, which had a beneficial effect on the corrosion resistance of coated steel by forming protective corrosion products in the wet/dry cyclic test.














Similar content being viewed by others
References
Lamaka, LV, Souto, RM, Ferreira, MGS, “In-situ Visualization of Local Corrosion by Scanning Ion-Selective Electrode Technique.” In: Mendez-Vilas, A, Diaz, J (eds.) Microscopy: Science, Technology, Applications and Education, Vol. 3. pp. 2162–2173 (2010)
Hausbrand, R, Stratmann, M, Rohwerder, M, “Corrosion of Zinc–Magnesium Coatings: Mechanism of Paint Delamination.” Corros. Sci., 51 2107–2114 (2009)
Maier, B, Frankel, GS, “Behavior of Magnesium-Rich Primers on AA2024-T3.” Corrosion, 67 055001-1–055001-15 (2011)
Battocchi, D, Simões, AM, Tallman, DE, Bierwagen, GP, “Electrochemical Behaviour of a Mg-rich Primer in the Protection of Al Alloys.” Corros. Sci., 48 1292–1306 (2006)
Simões, Alda, Battocchi, Dante, Tallman, Dennis, Bierwagen, Gordon, “Assessment of the Corrosion Protection of Aluminium Substrates by a Mg-rich Primer: EIS, SVET and SECM Study.” Prog. Org. Coat., 63 260–266 (2008)
Dong, CF, Sheng, H, An, YH, Li, XG, Xiao, K, Cheng, YF, “Corrosion of 7A04 Aluminum Alloy Under Defected Epoxy Coating Studied by Localized Electrochemical Impedance Spectroscopy.” Prog. Org. Coat., 67 269–273 (2010)
Sinebryukhov, SL, Gnedenkov, AS, Mashtalyar, DV, Gnedenkov, SV, “PEO-Coating/Substrate Interface Investigation by Localised Electrochemical Impedance Spectroscopy.” Surf. Coat. Technol., 205 1697–1705 (2010)
Maile, FJ, Schauer, T, Eisenbach, CD, “Evaluation of the Delamination of Coatings with Scanning Reference Electrode Technique.” Prog. Org. Coat., 38 117–120 (2000)
Akid, R, Mills, DJ, “A Comparison Between Conventional Macroscopic and Novel Microscopic Scanning Electrochemical Methods to Evaluate Galvanic Corrosion.” Corros. Sci., 43 1203–1216 (2001)
He, J, Gelling, VJ, Tallman, DE, Bierwagen, GP, “A Scanning Vibrating Electrode Study of Chromated Epoxy Primer on Steel and Aluminum.” J. Electrochem. Soc., 147 3661–3666 (2000)
Khobaib, M, Rensi, A, Matakis, T, Donley, MS, “Real Time Mapping of Corrosion Activity Under Coatings.” Prog. Org. Coat., 41 266–272 (2001)
Niu, L, Yin, Y, Guo, W, Lu, M, Qin, R, Chen, S, “Application of Scanning Electrochemical Microscopy in the Study of Corrosion of Metals.” J. Mat. Sci., 44 4511–4521 (2009)
Lister, TE, Pinhero, PJ, “Scanning Electrochemical Microscopy Study of Corrosion Dynamics on Type 304 Stainless Steel.” Electrochem. Solid St., 5 (11) B33–B36 (2002)
Fushimi, K, Seo, M, “An SECM Observation of Dissolution Distribution of Ferrous or Ferric Ion from a Polycrystalline Iron Electrode.” Electrochim. Acta, 47 121–127 (2001)
Fushimi, K, Lill, KA, Habazaki, H, “Heterogeneous Hydrogen Evolution on Corroding Fe–3 at% Si Surface Observed by Scanning Electrochemical Microscopy.” Electrochim. Acta, 52 4246–4253 (2007)
Lister, TE, Pinhero, PJ, “The Effect of Localized Electric Fields on the Detection of Dissolved Sulfur Species from Type 304 Stainless Steel Using Scanning Electrochemical Microscopy.” Electrochim. Acta, 48 2371–2378 (2003)
Lister, TE, Pinhero, PJ, “Microelectrode Array Microscopy: Investigation of Dynamic Behavior of Localized Corrosion at Type 304 Stainless Steel Surfaces.” Anal. Chem., 77 2601–2607 (2005)
Seegmiller, JC, Buttry, DA, “A SECM Study of Heterogeneous Redox Activity at AA2024 Surfaces Corrosion, Passivation, and Anodic Films.” J. Electrochem. Soc., 150 B413–B418 (2003)
Izquierdo, J, Santana, JJ, González, S, Souto, RM, “Uses of Scanning Electrochemical Microscopy for the Characterization of Thin Inhibitor Films on Reactive Metals: The Protection of Copper Surfaces by Benzotriazole.” Electrochim. Acta, 55 8791–8800 (2010)
González-García, Y, Santana, JJ, González-Guzmán, J, Izquierdo, J, González, S, Souto, RM, “Scanning Electrochemical Microscopy for the Investigation of Localized Degradation Processes in Coated Metals.” Prog. Org. Coat., 69 110–117 (2010)
Shao, Y, Jia, C, Meng, G, Zhang, T, Wang, F, “The Role of a Zinc Phosphate Pigment in the Corrosion of Scratched Epoxy-Coated Steel.” Corros. Sci., 51 371–379 (2009)
Huang, Y, Shih, H, Huang, H, Daugherty, J, Wu, S, Ramanathan, S, Chang, C, Mansfeld, F, “Evaluation of the Corrosion Resistance of Anodized Aluminum 6061 Using Electrochemical Impedance Spectroscopy (EIS).” Corros. Sci., 50 3569–3575 (2008)
Nordlien, JH, Sapa, AS, Ono, S, Masuko, N, Nisancioglu, K, “A TEM Investigation of Naturally Formed Oxide Films on Pure Magnesium.” Corros. Sci., 39 1397–1414 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xavier, J.R., Nishimura, T. Evaluation of the corrosion protection performance of epoxy coatings containing Mg nanoparticle on carbon steel in 0.1 M NaCl solution by SECM and EIS techniques. J Coat Technol Res 14, 395–406 (2017). https://doi.org/10.1007/s11998-016-9856-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9856-7