Abstract
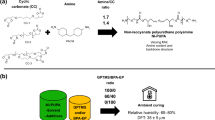
Glycidyl carbamate chemistry combines the excellent properties of polyurethanes with the crosslinking chemistry of epoxy resins. Glycidyl carbamate functional oligomers were synthesized by the reaction of polyfunctional isocyanate oligomers and glycidol. The oligomers were formulated into coatings with several amine functional crosslinkers at varying stoichiometric ratios and cured at different temperatures. Properties such as solvent resistance, hardness, and impact resistance were dependent on the composition and cure conditions. Most coatings had an excellent combination of properties. Studies were carried out to determine the kinetics of the curing reaction of the glycidyl carbamate functional oligomers with multifunctional and model amines. Detailed kinetic analysis of the curing reactions was also undertaken. The results indicated that the glycidyl carbamate functional group is more reactive than a glycidyl ether group.
Similar content being viewed by others
References
Hsia, H.C., Ma, C.C., Li, M.S., Li, Y.S., and Chen, D.S., “Glycidyl-Terminated Polyurethane Modified Epoxy Resins: Mechanical Properties, Adhesion Properties and Morphology”, J. Appl. Polym. Sci., 52, 1134 (1994).
Chean, C.S., and Woo, E.M., “Cure Kinetics and Morphology of Amine-Cured Tetraglycidyl-4,4′-diaminodiphenylmethane Epoxy Blends with Poly(Ether Imide)”, Polymer, 36 (15), 2883 (1995).
Schneider, N.S., Sprouse, J.F., Hagnauer, G.L., and Gillman, J.K., “DSC and TBA Studies of the Curing Behavior of Two Dicy-Containing Epoxy Resins”, Polym. Eng. Sci., 19 (4), 304 (1979).
Dusek, K., and Matejka, L., “Advances in Network Formation and Rheology During Curing of Epoxy Resin Systems Including Reactive Elastomers”, Polym. Mater. Sci. Eng., 57, 765 (1987).
Mijovic, J. and Wijaya, J., “Etherification Reactions in Epoxy-Amine Systems at High Temperature”, Polymer, 35 (12), 2683 (1994).
Matejka, L., and Dusek, K., “Mechanism and Kinetics of Curing of Epoxides Based on Diglycidylamine with Aromatic Amines. 2. The Reaction between Diglydidylaniline and Aniline”, Macromolecules, 22, 2911 (1988).
Duffy, J.V., Hui, E., and Hartman, B., “Reaction Kinetics for Hindered Amine/Epoxides by DSC”, J. Appl. Polym. Sci., 33 (8), 2959 (1987).
Buckley, L., and Roylance, D.K., “Kinetics of a Sterically Hindered Amine-Cured Epoxy Resin System”, Polym. Eng. Sci., 22 (3) (1982).
Johncock, P., Porecha, L., and Tudgey, G.F., “The Relative Reactivity of Primary and Secondary Amine Hydrogen Atoms of Aromatic Amines with Epichlorohydrin and N- and O-Glycidyl Compounds”, J. Polym. Sci., Polym. Chem. Ed., 23 (2), 291 (1985).
Wicks, Z. Jr., Jones, E.N., and Pappas, S.P., Organic Coatings and Science and Technology, Vol. 2, John Wiley and Sons, New York, 1992.
May, C.A. and Tanaka, Y. (Eds.), Epoxy Resins Chemistry and Technology, Vol. 2, Chapt. 3, Marcel Dekker, New York, 1988.
Ellis, B. (Ed.), Chemistry and Technology of Epoxy Resins, Vol. 1, Chapt. 1–2, Blackie Academic and Professional, New York, 1993.
Sourour, S. and Kamal, M.R., “Differential Scanning Calorimetry of Epoxy Cure: Isothermal Kinetics”, Thermochim. Acta, 14 (1–2), 41 (1976).
Ginsburg, D. (Ed.), Concerning Amines, Their Properties, Preparation and Reactions, Robert Maxwell, M.C., M.P. London, 1964.
Sung, C.S., Pyun, E., and Sun, H.L., “Characterization of Epoxy Cure by UV-Visible and Fluorescence Spectroscopy: Azochrophoric Labeling Approach,” Macromolecules, 19, 2922 (1986).
Matejka, L. and Dusek, K., “Mechanism and Kinetics of Curing of Epoxides Based on Diglycidylamine with Aromatic Amines. 1. The Reaction of Diglydidylaniline with Secondary Amines,” Macromolecules, 22, 2911 (1988).
Edwards, P., Erickson, J., and Webster, D.C., “Synthesis and Self-Crosslink-ing of Glycidyl Functional Oligomers,” Polym. Prepr., 44 (1), 54 (2003).
Edwards, P., Erickson, J., and Webster, D.C., “Synthesis and Characteriza-tion of Glycidyl Functional Oligomers,” Polym. Prepr., 44 (1), 144–145 (2003).
Cardillo, P. and Nebuloni, M., “Theoretical and Calorimetric Evaluation of Thermal Stability of Glycidol,” J. Loss Prevention in the Process Industries, 4 (4), 242 (1991).
Croll, S.G., “Atmospheric Gases and the Hardening of Amine-Cured Epoxy Coating,” Journal of Coatings Technology, 24, No. 664, 65 (1980).
Bell, J.P., Reffner, J.A., and Petrie, S., “Amine-Cured Epoxy Resins: Adhesion Loss Due to Reaction with Air,” J. Appl. Polym. Sci., 21 (4), 1095 (1977).
Grahm, J.P., Gloskey, D.A., Fisher, T.G., and Garling, R.A., “Effect of Temperature and Relative Humidity on Intercoat Adhesion Failure of Aliphatic Amine Cured Epoxy Coatings,” Journal of Coatings Technology, 60, No. 760, 35 (1988).
Edwards, P., Striemer, G., and Webster, D.C., “Kinetics and Cure of Glycidyl Carbamate Functional Oligomers,” Polym. Prepr., 90, 455 (2004).
Edwards, P., Striemer, G., and Webstet, D.C., “Cure Properties of Glycidyl Carbamate Oligomers Reacted with Amines,” Polym. Prepr., 45 (1), 935 (2004).
Caille, J.P., Pascault, J.P., and Tighzert, L., “Reaction of a Diepoxide with a Diisocyanate in Bulk,” Polym. Bull., 24 (1), 23 (1990).
Kordomenos, P.I., Frisch, K.C., and Kresta, J.E., “Oxazolidone Coatings. Part 2: Structure-Properties Relationships and Thermal Stability,” Journal of Coatings Technology, 55, No. 700, 59 (1983).
Ooi, S.K., Cook, W.D., Simon, G.P., and Such, C.H., “DSC Studies of the Curing Mechanisms and Kinetics of DGEBA using Imidazole Curing Agents,” Polymer, 41 (10), 3639 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edwards, P.A., Striemer, G. & Webster, D.C. Novel polyurethane coating technology through glycidyl carbamate chemistry. J Coat. Technol. Res. 2, 517–527 (2005). https://doi.org/10.1007/s11998-005-0011-0
Issue Date:
DOI: https://doi.org/10.1007/s11998-005-0011-0