Abstract
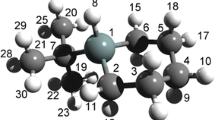
The equilibrium structure of tris(chloromethyl)amine, N(CH2Cl)3, has been determined in the gas phase using electron diffraction. Single-step distance corrections (representing the differences between the interatomic distances from the equilibrium structure and those from the vibrationally averaged structure) and amplitudes of vibration have been computed using semi-empirical molecular dynamics (SE-MD) simulations in order to treat accurately the description of the low-frequency, large-amplitude vibrational modes associated particularly with one CH2Cl group. A series of complementary theoretical calculations using the SOGGA11-X DFT functional with correlation-consistent basis sets of double-, triple-, and quadruple-ζ quality is also presented. The agreement between the experimental and theoretical equilibrium structural parameters attests to the accuracy of the applied theoretical calculations and of our gas-phase structural solution. Raman spectra have been recorded over a range of temperatures, allowing the solid and the melt to be studied, and the Raman-active intramolecular modes to be identified. Free from the influence of intermolecular interaction, the structure of tris(chloromethyl)amine in the gas phase is markedly different to that reported in the literature for the single crystal. This is discussed, and evidence for the anomeric effect in tris(chloromethyl)amine is evaluated.










Similar content being viewed by others
References
Klapötke TM, Krumm B, Scherr M, Steemann FX, Banert K, Joo YH (2009) Experimental and theoretical studies on some energetic functionalized trimethylamine derivatives. Chem Eur J 15:11341–11345
Livingston RL, Vaughan G (1956) The molecular structure of perfluorotrimethylamine by electron diffraction. J Am Chem Soc 78:4866–4869
Bürger H, Niepel H, Pawelke G (1979) Vibrational spectra and normal coordinate analysis of CF3 compounds: part XXVII. Perfluorotrimethylamine. Reinvestigation of the molecular structure by electron diffraction. J Mol Struct 54:159–174
Dimitrov A, Mack HG, Rüdiger S, Seppelt K, Oberhammer H (1994) Structure and conformation of tris(2,2,2-trifluoroethyl)amine, N(CH2CF3)3, in the gaseous and solid state. J Phys Chem A 98:11401–11405
Beagley B, Medwid AR (1977) Vibrational force fields and amplitudes and zero-point average structures of (CH3)3Y molecules (Y = N, P, As, Sb, Bi): a combination of electron-diffraction and spectroscopic data. J Mol Struct 38:229–238
Higginbotham HK, Bartell LS (1965) Electron diffraction study of CH3NH2 and CD3ND2. J Chem Phys 42:1131–1132
Beagley B, Hewitt TG (1968) Electron diffraction study of gaseous dimethylamine and trimethylamine. Trans Faraday Soc 64:2561–2570
Iijima T, Jimbo H, Taguchi M (1986) The molecular structure of methylamine in the vapour phase. J Mol Struct 144:381–383
Takeuchi H, Kojima T, Egawa T, Konaka S (1992) Molecular structure and conformations of diethylamine and triethylamine as determined by gas electron diffraction, ab initio calculations and vibrational spectroscopy. J Phys Chem A 96:4389–4396
Nishikawa T, Itoh T, Shimoda K (1955) Molecular structure of methylamine from its microwave spectrum. J Chem Phys 23:1735–1736
Lide Jr DR (1957) Structure of the methylamine molecule. I. Microwave spectrum of CD3ND2. J Chem Phys 27:343–352
Lide Jr DR (1958) Microwave spectra of molecules exhibiting internal rotation. III. Trimethylamine. J Chem Phys 28:572–576
Kumar K (1971) Rotational isomerism in triethylamine. Chem Phys Lett 9:504–507
Crocker, C, Goggin, PL (1978) Infrared and Raman spectroscopic studies of conformations in liquid and solid triethyl-, diethyl(methyl)- and ethyldimethyl-amines, -phosphines and -arsines. J Chem Soc Dalton Trans 388–394
Bushweller CH, Fleischman SH, Grady GL, McGoff P, Rithner CD, Whalon MR, Brennan JG, Marcantonio RP, Dominigue RP (1982) Stereodynamics of diethylmethylamine and triethylamine. J Am Chem Soc 104:6224–6236
Lemieux RU (1971) Newer developments in the conformational analysis of carbohydrates. Pure Appl Chem 27:527–548
Edward JT (1955) Stability of glycosides to acid hydrolysis. Chem Ind:1102–1104
Oberhammer H (2006) Anomeric effect in the N-C-F moiety. Mendeleev Commun 16:136–137
Christen D, Mack HG, Rüdiger S, Oberhammer H (1996) The anomeric effect in (fluoromethyl)dimethylamine, CH2FN(CH3)2. J Am Chem Soc 118:3720–3723
Jin AD, Zhu XL, Kirchmeier RL, Shreeve JM, Patel NR, Oberhammer H (1994) The effect of fluorination in trimethylamine—gas-phase structures of CF3N(CH3)2 and (CF3)2NCH3. J Mol Struct 323:129–134
Minkwitz R, Lamek D, Korn M, Oberhammer H (1993) On the gas phase structure of CF3NCl2 and the preparation of CF3NCl2F+MF6 − (M = As, Sb) and CF2=NClF+SbF6 −. Anorg Allg Chem 619:2066–2070
Minkwitz R, Lamek D, Korn M, Oberhammer H (1994) The gas phase structure of CF3NBr2 and (CF3)2NBr. Anorg Allg Chem 620:353–356
Bürger H, Pawelke G, Oberhammer H (1982) Vibrational spectra and normal coordinate analysis of CF3 compounds part XLI. Vibrational spectra, normal coordinate analysis and electron diffraction investigation of hexafluoroazomethane and cis- and trans-1,1,1,4,4,4-hexafluorobut-2-ene. J Mol Struct 84:49–68
Bürger H, Pawelke G, Oberhammer H (1985) Vibrational spectra and normal coordinate analysis of CF3 compounds part XLVI. Synthesis, vibrational spectra, normal coordinate analysis and electron diffraction investigation of the cis isomer of hexafluoroazomethane. J Mol Struct 128:283–295
Dorofeeva OV, Mitin AV, Altova EP, Karasev NM, Nabiev OG, Vilkov LV, Oberhammer H (2011) Anomeric effect in N-aziomethylpyrrolidine: gas-phase electron diffraction and theoretical study. Phys Chem Chem Phys 13:1490–1498
Radom L, Hehre WJ, Pople JA (1972) Molecular orbital theory of the electronic structure of organic compounds XIII. Fourier component analysis of internal rotation potential functions in saturated molecules. J Am Chem Soc 94:2371–2381
Schleyer PR, Kos AJ (1983) The importance of negative (anionic) hyperconjugation. Tetrahedron 39:1141–1150
Schleyer PR, Jemmis ED, Spitznagel GW (1985) Do anomeric effects involving the second-row substituents, chlorine, mercapto and phosphino exist? Stabilization energies and structural preferences. J Am Chem Soc 107:6393–6394
Reed AE, Schleyer PR (1988) The anomeric effect with central atoms other than carbon: strong interactions between nonbonded substituents in mono- and polyfluorinated first and second-row amines. Inorg Chem 27:3969–3987
Rastelli A, Cocchi M (1991) Model calculations of chemical interactions part 3: rotational energy profiles in simple molecules: evaluation, additivity and role of bond-bond, bond-lone-pair and lone-pair-lone-pair interactions. J Chem Soc Faraday Trans 87:249–258
Szinicz L (2005) History of chemical and biological warfare agents. Toxicology 214:167–181
Fraga CG, Bronk K, Dockendorff BP, Heredia-Langner A (2016) Organic chemical attribution signatures for the sourcing of a mustard agent and its starting materials. Anal Chem 88:5406–5413
Centers for Disease Control and Prevention. Facts about nitrogen mustards. https://emergency.cdc.gov/agent/nitrogenmustard/basics/facts.asp. Accessed 30 Oct 2016
Organization for the Prohibition of Chemical Weapons. Annex of chemicals. https://www.opcw.org/chemical-weapons-convention/annexes/annex-on-chemicals. Accessed 30 Oct 2016
Mitzel NW, Brown DH, Parsons S, Brain PT, Pulham CR, Rankin DWH (1998) Differences between gas-phase and solid-state molecular structures of the simplest phosphonium ylide, Me3P=CH2. Angew Chem Int Ed 37:1670–1672
Mitzel NW, Lustig C, Berger RJF, Runeberg N (2002) Luminescence phenomena and solid-state structures of trimethyl and triethylgallium. Angew Chem Int Ed 41:2519–2522
Mitzel NW, Vojinovic K, Foerster T, Robertson HE, Borisenko KB, Rankin DWH (2005) (Dimethylaminomethyl)trifluorosilane, Me2NCH2SiF3—a model for the α-effect in aminomethylsilanes. Chem Eur J 11:5114–5125
Hagemann M, Mix A, Berger RJF, Pape T, Mitzel NW (2008) Strong intramolecular Si-N interactions in the chlorosilanes Cl3-nHnSiOCH2CH2NMe2 (n = 1-3). Inorg Chem 47:10554–10564
Hagemann M, Berger RJF, Hayes SA, Stammler HG, Mitzel NW (2008) N,N-Dimethylaminopropylsilane: a case study on the nature of weak intramolecular Si···N interactions. Chem Eur J 14:11027–11038
Fluck E, Meiser P (1971) Preparation of tris(chloromethyl)amine, N(CH2Cl)3. Angew Chem Int Ed Engl 10:653
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Revision D.01. Gaussian Inc, Wallingford
CP2K Developers’ Group (2015) CP2K v.3.0
York Advanced Research Computing Cluster. http://www.york.ac.uk/it-services/services/yarcc/
EPSRC National Service for Computational Chemistry Software. http://www.nsccs.ac.uk/
Peverati R, Truhlar DG (2011) A global hybrid generalized gradient approximation to the exchange-correlation functional that satisfies the second-order density-gradient constraint and has broad applicability in chemistry. J Chem Phys 135:191102
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Woon DE, Dunning Jr TH (1993) Gaussian basis sets for use in correlated molecular calculations III. The atoms aluminium through argon. J Chem Phys 98:1358–1371
Peng C, Schlegel HB (1993) Combining synchronous transit and quasi-Newton methods for finding transition states. Israel J Chem 33:449–454
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J Comp Chem 17:49–56
Mitzel NW, Smart BA, Blake AJ, Robertson HE, Rankin DWH (1996) Conformational analysis of 1,4-disilabutane and 1, 5-disilapentane by combined application of gas-phase electron diffraction and ab initio calculations and the crystal structure of 1, 5-disilapentane at low temperatures. J Phys Chem A 100:9339–9347
Blake AJ, Brain PT, McNab H, Miller J, Morrison CA, Parsons S, Rankin DWH, Robertson HE, Smart BA (1996) Structure analysis restrained by ab initio calculations: the molecular structure of 2, 5-dichloropyrimidine in gaseous and crystalline phases. J Phys Chem A 100:12280–12287
Mitzel NW, Rankin DWH (2003) SARACEN—molecular structures from theory and experiment: the best of both worlds. Dalton Trans:3650–3662
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785–789
Perdew JP (1986) Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B 33:8822–8824
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules solids and surfaces applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Wann DA, Less RJ, Rataboul F, McCaffrey PD, Reilly AM, Robertson HE, Lickiss PD, Rankin DWH (2008) Accurate gas-phase experimental structures of octasilsesquioxanes (Si8O12X8; X = H, Me). Organometallics 27:4183–4187
Wann DA, Reilly AM, Rataboul F, Lickiss PD, Rankin DWH (2009) The gas-phase structure of the hexasilsesquioxane Si6O9(OSiMe3)6. Z Naturforsch 64:1269–1275
Wann DA, Zakharov AV, Reilly AM, McCaffrey PD, Rankin DWH (2009) Experimental equilibrium structures: application of molecular dynamics simulations to vibrational corrections for gas electron diffraction. J Phys Chem A 113:9511–9520
Wann DA, Dickson CN, Lickiss PD, Robertson HE, Rankin DWH (2011) The gas-phase equilibrium structures of Si8O12(OSiMe3)8 and Si8O12(CHCH2)8. Inorg Chem 50:2988–2994
Huntley CM, Laurenson GS, Rankin DWH (1980) Gas-phase molecular structure of bis(difluorophosphino)amine, determined by electron diffraction. J Chem Soc Dalton Trans:954–957
Fleischer H, Wann DA, Hinchley SL, Borisenko KR, Lewis JR, Mawhorter RJ, Robertson HE, Rankin DWH (2005) Molecular structures of Se(SCH3)2 and Te(SCH3)2 using gas-phase electron diffraction and ab initio and DFT geometry optimisations. Dalton Trans:3221–3228
Hinchley SL, Robertson HE, Borisenko KR, Turner AR, Johnston BF, Rankin DWH, Ahmadian M, Jones JN, Cowley AH (2004) The molecular structure of tetra-tert-butyldiphosphine: an extremely distorted, sterically crowded molecule. Dalton Trans:2469–2476
Ross AW, Fink M, Hilderbrandt R (1992) International tables for crystallography. Kluwer Academic Publishers, Dordrecht
Masters SL, Atkinson SJ, Hölbling M, Hassler K (2013) Gas-phase molecular structure of 1,1,1,2-tetrabromo-2,2-dimethyldisilane: theoretical and experimental investigation of a super-halogenated disilane and computational investigation of the F, Cl and I analogues. Struct Chem 24:1201–1206
Møllendal H, Samdal S, Guillemin J (2011) Microwave spectrum, conformational composition and intermolecular hydrogen bonding of (2-chloroethyl)amine (ClCH2CH2NH2). J Phys Chem A 115:4334–4341
Alecu IM, Zheng J, Zhao Y, Truhlar DG (2010) Computational thermochemistry: scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries. J Chem Theory Comput 6:2872–2887
Acknowledgments
C.D.R thanks João Pedro Nunes for useful discussions on data extraction and provision of the data extraction package xstract. We extend our thanks the staff at the York Advanced Research Computing Cluster (YARCC) and the UK National Service for Computational Chemistry Software (NSCCS) for the provision of computational resources.
Funding
D.A.W and C.D.R thank the EPSRC for funding the gas electron diffraction and theoretical research at the University of York, UK, for funding a fellowship for D.A.W. (EP/I004122), and for funding the studentship of C.D.R. S.J.A thanks the Department of Chemistry, University of Canterbury, NZ, for funding a studentship, and for the award of the inaugural Betty Wignall Scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No human or animal studies were conducted as part of this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
Supporting Information: Summary of parameters for the GED data collection (Table S1); the Cartesian coordinates and energies of A and B as optimized at the SOGGA11-X level (Tables S2–S4); the Cartesian coordinates and energies of TS A,B as optimized at the SOGGA11-X level (Tables S5–S7); summary of parameters for the GED data refinement (Table S8); least-squares correlation matrix for the GED data refinement (Table S9); the Cartesian coordinates and energies of A as optimized at the B3LYP (Tables S10–S12), B3P86 (Tables S13–S15), B3PW91 (Tables S16–S18) and wB97XD (Tables S19–S21) levels; the Cartesian coordinates and energies of methenamine as optimized at the SOGGA11-X level (Tables S22–S24); details of the molecular model used in the least-squares refinement procedure; refined parameters, and SARACEN restraints (Table S25); interatomic distances, GED-determined and theoretical amplitudes of vibration, and MD-derived distance corrections (Table S26); the refined GED structure of TCMA in Cartesian coordinates (Table S27). (DOCX 131 kb)
Rights and permissions
About this article
Cite this article
Rankine, C.D., Atkinson, S.J., Waterland, M.R. et al. The structure of tris(chloromethyl)amine in the gas phase using quantum chemical calculations and gas electron diffraction and as a solid and melt using Raman spectroscopy. Struct Chem 29, 803–813 (2018). https://doi.org/10.1007/s11224-018-1089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1089-1