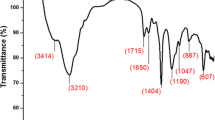
Abstract
Monomers composed of a polymerizable methacrylate moiety connected to a short poly(ethylene) glycol (PEG) chain are versatile building-blocks for the preparation of “smart” biorelevant materials. Hydrogels based on these PEG methacrylates are a very important class of biomaterials with several applications. The radical polymerization kinetics of two such oligomers, namely poly(ethylene glycol) methacrylate (PEGMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMEMA) was investigated. Experimental polymerization rate and monomer conversion data were measured using DSC operating under non-isothermal conditions, at several constant heating rates, or isothermal, at different constant reaction temperatures. Isoconversional techniques were employed to estimate the variation of the polymerization effective activation energy as a function of the extent of reaction. It was found that isothermal and non-isothermal experiments results in similar trends of the activation energy, whereas by comparison of differential to integral isoconversional methods it was postulated that in non-isothermal polymerization experiments integral methods should not be used. From comparison of the two oligomers employed in the polymerization experiments, it was clear that the presence of the terminal hydroxyl group in PEGMA compared to the methoxy group in PEGMEMA leads to different conversion time profiles and activation energies. In particular, monomer–monomer association through hydroxyl groups results in initially lower activation energy of PEGMA. As polymerization proceeds, the existence of aggregated hydroxyl structures (···OH···OH···OH···) in the PEGMA macromolecular chains result in higher activation energies and a more abrupt increase in the conversion time curve.









Similar content being viewed by others
References
Achilias DS, Nikolaidis AK, Karayannidis GP. PMMA/organomodified montmorillonite nanocomposites prepared by in situ bulk polymerization. Study of the reaction kinetics. J Therm Anal Calorim. 2010;102(2):451–60.
Achilias DS, Verros GD. Modeling of diffusion-controlled reactions in free radical solution and bulk polymerization: model validation by DSC experiments. J Appl Polym Sci. 2010;116:1842–56.
Achilias DS, Sideridou I. Kinetics of the benzoyl peroxide/amine initiated free-radical polymerization of dental dimethacrylate monomers: experimental studies and mathematical modelling for TEGDMA and Bis-EMA. Macromolecules. 2004;37:4254–65.
Viciosa MT, Quiles Hoyo J, Dionisio M, Gomez Ribelles JL. Temperature modulated DSC study of the kinetics of free radical isothermal network polymerization. J Therm Anal Calorim. 2007;90:407–14.
Ye G, Cui Y, Liu X, Zhou H. Kinetic investigation of photopolymerization initiated by oligomeric photolatent base. J Therm Anal Calorim. 2013;112:1499–506.
Passos MF, Dias DRC, Bastos GNT, Jardini AL, Benatti ACB, Dias CGBT, Filho Maciel R. PHEMA hydrogels. J Therm Anal Calorim. 2016;125:361–8.
Achilias DS. A review of modelling of diffusion-controlled polymerization reactions. Macromol Theory Simul. 2007;16:319–47.
Achilias DS. Investigation of the radical polymerization kinetics using DSC and mechanistic or isoconversional methods. J Therm Anal Calorim. 2014;116:1379–86.
Lutz J-F. Polymerization of oligo(ethylene glycol) (meth)acrylates: toward new generations of smart biocompatible materials. J Polym Sci A Polym Chem. 2008;46:3459–70. https://doi.org/10.1002/pola.22706.
Lutz JF, Hoth A, Schade K. Design of oligo(ethylene glycol)-based thermoresponsive polymers: an optimization study. Des Monom Polym. 2009;12(4):343–53.
Lutz J-F, Hoth A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules. 2006;39(2):893–6. https://doi.org/10.1021/ma0517042.
Üzgün S, Akdemir O, Hasenpusch G, Maucksch C, Golas MM, Sander B, Stark H, Imker R, Lutz J-F, Rudolph C. Characterization of tailor-made copolymers of oligo(ethylene glycol) methyl ether methacrylate and N,N-dimethylaminoethyl methacrylate as nonviral gene transfer agents: influence of macromolecular structure on gene vector particle properties and transfection efficiency. Biomacromolecules. 2010;11(1):39–50. https://doi.org/10.1021/bm9008759.
Lutz J-F. Thermo-switchable materials prepared using the OEGMA-platform. Adv Mater. 2011;23:2237–43. https://doi.org/10.1002/adma.201100597.
Achilias DS, Siafaka PI. Polymerization kinetics of poly(2-hydroxyethyl methacrylate) hydrogels and nanocomposite materials. Processes. 2017;5(2):21. https://doi.org/10.3390/pr5020021.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Cham: Springer; 2015.
Matyjaszewski K, Davis TP. Handbook of radical polymerization. Hoboken: Wiley; 2002.
Morita S. Hydrogen-bonds structure in poly(2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy. Front Chem. 2014;2:1–5.
Liang K, Hutchinson RA. Solvent effects on free-radical copolymerization propagation kinetics of styrene and methacrylates. Macromolecules. 2010;43:6311–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achilias, D.S., Tsagkalias, I.S. Investigation of radical polymerization kinetics of poly(ethylene glycol) methacrylate hydrogels via DSC and mechanistic or isoconversional models. J Therm Anal Calorim 134, 1307–1315 (2018). https://doi.org/10.1007/s10973-018-7535-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7535-x