Abstract
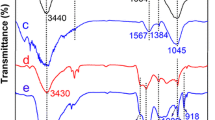
A new phosphorylated mesoporous carbon (CMK-3-PO4) was synthesized by situ phosphorylation of concentrated phosphoric acid (85 %) using mesoporous carbon (CMK-3) as a template. The maximum monolayer adsorption capacity of adsorbents increased from 133.5 mg g−1 (CMK-3) to 485.4 mg g−1 (CMK-3-PO4) due to the extended oxygen functional groups, and the U(VI) adsorption on CMK-3-PO4 was endothermic and spontaneous in nature. The selective sorption ability of U(VI) was significantly improved after phosphorylation. The U(VI) in the CMK-3-PO4 could been eluted by 1.0 mol L−1 HCl and also had good reusing property, and this may offer the CMK-3-PO4 very promising application prospects.













Similar content being viewed by others
References
Anirudhan TS, Radhakrishnan PG (2009) Improved performance of a biomaterialbased cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100:250–257
ElSweify FH, Shehata MKK, ElShazly EAA (1995) Ion exchange studies of Arsenazo-III and thorium (IV) from aqueous solutions of Arsenazo-III with AG-2 × 8, Dowex-50 W × 8 and Chelex-100. J Radioanal Nucl Chem 198:77–87
Kumari N, Prabhu D, Pathak P, Kanekar A, Manchanda V (2011) Extraction studies of uranium into a third-phase of thorium nitrate employing tributyl phosphate and N, N-dihexyl octanamide as extractants in different diluents. J Radioanal Nucl Chem 289:835–843
Goldberg S, Criscenti LJ, Turner DR (2007) Modeling adsorption of metals and metalloids by soil components. Vadose Zone J 6:407–435
Ryoo R, Joo SH, Jun S (1999) Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J Phys Chem B 103:7743–7746
He J, Ma K, Jin J (2009) Preparation and characterization of octyl-modified ordered mesoporous carbon CMK-3 for phenol adsorption. Microporous Mesoporous Mater 121:173–177
Liu G, Zheng S, Yin D (2006) Adsorption of aqueous alkylphenol ethoxylate surfactants by mesoporous carbon CMK-3. J Colloid Interface Sci 302:47–53
Kruk M, Jaroniec M, Kim TW, Ryoo R (2003) Synthesis and characterization of hexagonally ordered carbon nanopipes. Chem Mater 15:2815–2823
Darmstadt H, Roy C, Kaliaguine S, Kim TW, Ryoo R (2003) Surface and pore structures of CMK-5 ordered mesoporous carbons by adsorption and surface spectroscopy. Chem Mater 15:3300–3307
Fan J, Yu C, Gao F (2003) Cubic Mesoporous Silica with Large Controllable Entrance Sizes and Advanced Adsorption Properties. Angew Chem Int Ed 42:3146–3150
Vinu A, Miyahara M, Sivamurugan V (2005) Large pore cage type mesoporous carbon, carbon nanocage: a superior adsorbent for biomaterials. J Mater Chem 15:5122–5127
Shuang C, Pan F, Zhou Q, Zhang M, Li A, Li P (2013) Adsorption of HA fractions with different molecular weight on magnetic polyacrylic anion exchange resin. Funct Nat Org Matter Changing Environ 01:177–180
Caldas EM, Menezes EW, Pizzolato TM (2014) Ionic silsesquioxane film immobilized on silica applied in the development of carbon paste electrode for determination of methyl parathion. J Sol-Gel Sci Technol 72:282–289
Behbahani M, Tapeh NAG, Mahyari M (2014) Monitoring of trace amounts of heavy metals in different food and water samples by flame atomic absorption spectrophotometer after preconcentration by amine-functionalized graphene nanosheet. Environ Monit Assess 186:7245–7257
Samonin VV, Nikonova VY, Spiridonova EA (2007) The influence of the preliminary adsorption of water on the adsorption of organic solvent vapors on fullerene materials. Russ J Phys Chem A 81:1271–1275
Pandyan RK, Seenithurai S, Mahendran M (2012) Carbon monoxide adsorption on transition element-doped single wall carbon nanotube. Indian J Phys 86:677–680
Vinu A, Hartmann M (2005) Characterization and microporosity analysis of mesoporous carbon molecular sieves by nitrogen and organics adsorption. Catal Today 102–103:189–196
Vinu A, Hossian KZ, Srinivasu P (2007) Carboxy-mesoporous carbon and its excellent adsorption capability for proteins. J Mater Chem 17:1819–1825
Haque E, Khan NA, Talapaneni SN (2010) Adsorption of phenol on mesoporous carbon CMK-3: effect of textural properties. Bull Korean Chem Soc 31:1638–1642
Wu Z, Webley PA, Zhao D (2010) Comprehensive study of pore evolution, mesostructural stability, and simultaneous surface functionalization of ordered mesoporous carbon (FDU-15) by wet oxidation as a promising adsorbent. Langmuir 26:10277–10286
Yue L, Smart NG, Chien W (1995) Supercritical fluid extraction of uranium and thorium from nitric acid solutions with organophosphorus reagents. Environ Sci Technol 29:2706–2708
Brian TH, Christopher FB, Thang D, Andreas S (1999) Synthesis of highly ordered, three-dimensional, macroporous structures of amorphous or crystalline inorganic oxides, phosphates, and hybrid composites. Chem Mater 11:795–805
Yu X, Liu Y, Zhou Z, Xiong G, Cao X, Li M, Zhang Z (2014) Adsorptive removal of U(VI) from aqueous solution by hydrothermal carbon spheres with phosphate group. J Radioanal Nucl Chem 300:1235–1244
Lee J-S, Joo SH, Ryoo R (2002) Synthesis of mesoporous silicas of controlled pore wall thickness and their replication to ordered nanoporous carbons with various pore diameters. J Am Chem Soc 124:1156–1157
Vinu A, Hossain KZ, Kumar SG, Ariga K (2006) Adsorption of l-histidine over mesoporous carbon molecular sieves. Carbon 44:530–536
Barrett EP, Joyner L, Halenda P (1952) Granular adsorbents for sugar refining. Some factors affecting porosity and activity in service. Ind Eng Chem 44:1827–1833
Nie B, Zhang Z, Cao X, Liu Y, Liang P (2013) Sorption study of uranium from aqueous solution on ordered mesoporous carbon CMK-3. J Radioanal Nucl Chem 295:663–670
Zhang F, Meng Y, Gu D, Yu C, Tu B, Zhao D (2005) A facile aqueous route to synthesize highly ordered mesoporous polymers and carbon frameworks with Ia3d bicontinuous cubic structure. J Am Chem Soc 127:13508–13509
Eitan A, Jiang K, Dukes D, Andrews R, Schadler LS (2003) Surface modification of multiwalled carbon nanotubes: toward the tailoring of the interface in polymer composites. Chem Mater 16:3198–3201
Wang Y, Zhang Z, Liu Y, Cao X, Liu Y, Li Q (2012) Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem Eng J 198–199:246–253
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium (II) and barium (II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Psareva T, Zakutevskyy O, Chubar N, Strelko V, Shaposhnikova T, Carvalho JR, Correia M (2005) Uranium sorption on cork biomass. Colloids Surf A 252:231–236
Anirudhan T, Radhakrishnan P (2009) Improved performance of a biomaterial-based cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100:250–257
Hazer O, Kartal S (2010) Use of amidoximated hydrogel for removal and recovery of U(VI) ion from water samples. Talanta 82:1974–1979
Jaroniec M (1977) Adsorption of gas mixtures on heterogeneous solid surfaces II. Adsorption isotherms for gaseous mixtures whose pure-gas isotherms show the Freundlich, Tóth and Langmuir behaviours. Colloid Polym Sci 255:32–34
Fuertes Antonio B (2001) Preparation and characterization of adsorption-selective carbon membranes for gas separation. Adsorption 7:117–129
Anirudhan T, Divya L, Suchithra P (2009) Kinetic and equilibrium characterization of uranium (VI) adsorption onto carboxylate-functionalized poly (hydroxyethylmethacrylate)-grafted lignocellulosics. J Environ Manage 90:549–560
Zhang Z, Yu X, Cao X, Hua R, Li M, Liu Y (2014) Adsorption of U(VI) from aqueous solution by sulfonated ordered mesoporous carbon. J Radioanal Nucl Chem 301:821–830
Liu Y, Wang Y, Zhang Z, Cao X, Nie W, Li Q, Hua R (2013) Removal of uranium from aqueous solution by a low cost and high-efficient adsorbent. Appl Surf Sci 273:68–74
Liu Y, Cao X, Hua R, Wang Y, Liu Y, Pang C, Wang Y (2010) Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 02:150–155
Acknowledgments
This work is financially supported by National Key Basic Research Development Program (973 Program) Project of China (Grant No. 2014CB460604), National Natural Science Foundation of China (Grant Nos. 21101024, 21201033, 21301028), National Undergraduate Training Programs for Innovation and Entrepreneurship (Grant No. 201210405006), Key Project of Chinese Ministry of Education (Grant No. 211086), the Young Scientists Training Program of Jiangxi Province (Grant No. 20122BCB23023), Natural Science Foundation of Jiangxi Province (Grant Nos. 20114BAB203002, 20122BAB203012, 20132BAB203027), China Postdoctoral Science Foundation (Grant No. 20110490857), and Project of Jiangxi Provincial Department of Education (Grant No. GJJ13452) and Open Project Foundation of Fundamental Science on Radioactive Geology and Exploration Technology Laboratory (East China Institute of Technology) (Grant No. RGET1311).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, YD., Cao, XH., Luo, XP. et al. Recycle of U(VI) from aqueous solution by situ phosphorylation mesoporous carbon. J Radioanal Nucl Chem 306, 515–525 (2015). https://doi.org/10.1007/s10967-015-4133-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4133-2