Abstract
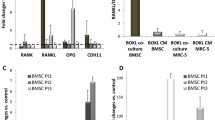
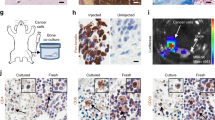
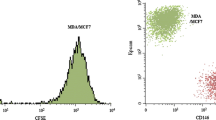
The preferential metastasis of breast cancer cells to bone is a complex set of events including homing and preferential growth which may include unique factors produced by bone cells in the immediate microenvironment. In this study, we evaluated the suitability of bone cells derived from orthoplastic surgeries for use in an in vitro co-culture system representing a model of the bone microenvironment. Using a limiting dilution assay we determined the relative survival and proliferation potentials of breast cancer cell lines co-cultured on bone-derived cells or on Hs68 fibroblasts. The comparison of bone and skin fibroblastic substrata indicates that MCF-7 cells preferentially survive and grow in a bone microenvironment (P < 0.001). Overall, we show that bone-derived cells enhance survival, proliferation, and migration of breast cancer cells, where migration is in part mediated by bone cell-produced osteopontin. Our in vitro co-culture model system provides a robust cost-effective method to study the various factors that mediate cancer/bone-derived cell interactions.





Similar content being viewed by others
References
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55:61–66
Fokas E, Engenhart-Cabillic R, Daniilidis K, Rose F, An HX (2007) Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 26:705–715
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2:584–593
Rabbani SA, Mazar AP (2007) Evaluating distant metastases in breast cancer: from biology to outcomes. Cancer Metastasis Rev 26:663–674
Paget S (1889) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 8:98–101
Kaplan RN, Psaila B, Lyden D (2006) Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev 25:521–529
Tuck AB, Chambers AF (2001) The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia 6:419–429
Cook AC et al (2005) Osteopontin induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol Carcinog 43:225–236
Coppola D et al (2004) Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res 10:184–190
Rudland PS et al (2002) Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res 62:3417–3427
Fedarko NS, Jain A, Karadag A (2001) Van Eman, M.R. & Fisher, L.W. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res 7:4060–4066
Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF (2000) Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J Cell Biochem 78:465–475
Singhal H et al (1997) Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res 3:605–611
Tuck AB et al (1999) Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 18:4237–4246
Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF (2001) The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med 1:621–632
Chambers AF (2009) MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res 69:5292–5293
Christgen M, Lehmann U (2007) MDA-MB-435: the questionable use of a melanoma cell line as a model for human breast cancer is ongoing. Cancer Biol Ther 6:1355–1357
Ellison G et al (2002) Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol 55:294–299
Lacroix M (2009) MDA-MB-435 cells are from melanoma, not from breast cancer. Cancer Chemother Pharmacol 63:567
Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD (2007) MDA-MB-435 cells are derived from M14 melanoma cells—a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 104:13–19
Ross DT et al (2000) Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24:227–235
Sellappan S et al (2004) Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res 64:3479–3485
Rastaldi MP et al (2002) Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62:137–146
Davila RM, Crouch EC (1993) Role of mesothelial and submesothelial stromal cells in matrix remodeling following pleural injury. Am J Pathol 142:547–555
Lefkovits I, Waldmann H (1999) Limiting dilution analysis of cells of the immune system. Oxford University Press, New York
Laemmli K (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Wagner W, Ho AD (2007) Mesenchymal stem cell preparations—comparing apples and oranges. Stem Cell Rev 3:239–248
Wagner W et al (2007) Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells 25:2638–2647
Kapoor P, Suva LJ, Welch DR, Donahue HJ (2008) Osteoprotegrin and the bone homing and colonization potential of breast cancer cells. J Cell Biochem 103:30–41
Mercer RR, Mastro AM (2005) Cytokines secreted by bone-metastatic breast cancer cells alter the expression pattern of f-actin and reduce focal adhesion plaques in osteoblasts through PI3 K. Exp Cell Res 310:270–281
Mazzali M et al (2002) Osteopontin—a molecule for all seasons. QJM 95:3–13
Denhardt DT, Guo X (1993) Osteopontin: a protein with diverse functions. FASEB J 7:1475–1482
Rittling SR, Chambers AF (2004) Role of osteopontin in tumour progression. Br J Cancer 90:1877–1881
Wai PY, Kuo PC (2004) The role of Osteopontin in tumor metastasis. J Surg Res 121:228–241
Giachelli CM, Steitz S (2000) Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol 19:615–622
Frolke JP et al (2004) Viable osteoblastic potential of cortical reamings from intramedullary nailing. J Orthop Res 22:1271–1275
Kaur D, Berger P, Duffy SM, Brightling CE, Bradding P (2005) Co-cultivation of mast cells and Fc epsilon RI alpha + dendritic-like cells from human hip bone marrow. Clin Exp Allergy 35:226–233
Tydings JD, Martino LJ, Kircher M, Alfred RH, Lozman J (1987) Viability of intramedullary canal bone reamings for continued calcification. Am J Surg 153:306–309
Lodie TA et al (2002) Systematic analysis of reportedly distinct populations of multipotent bone marrow-derived stem cells reveals a lack of distinction. Tissue Eng 8:739–751
Hughes L, Malone C, Chumsri S, Burger AM, McDonnell S (2008) Characterisation of breast cancer cell lines and establishment of a novel isogenic subclone to study migration, invasion and tumourigenicity. Clin Exp Metastasis 25:549–557
Plopper GE, Domanico SZ, Cirulli V, Kiosses WB, Quaranta V (1998) Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res Treat 51:57–69
Zhang X et al (2004) Motility response to insulin-like growth factor-I (IGF-I) in MCF-7 cells is associated with IRS-2 activation and integrin expression. Breast Cancer Res Treat 83:161–170
Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H (2007) The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev 16:1087–1097
Noti JD (2000) Adherence to osteopontin via alphavbeta3 suppresses phorbol ester-mediated apoptosis in MCF-7 breast cancer cells that overexpress protein kinase C-alpha. Int J Oncol 17:1237–1243
Tanabe KK, Nishi T, Saya H (1993) Novel variants of CD44 arising from alternative splicing: changes in the CD44 alternative splicing pattern of MCF-7 breast carcinoma cells treated with hyaluronidase. Mol Carcinog 7:212–220
He B, Mirza M, Weber GF (2005) An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene 25:2192–2202
Adwan H, Bauerle TJ, Berger MR (2004) Downregulation of osteopontin and bone sialoprotein II is related to reduced colony formation and metastasis formation of MDA-MB-231 human breast cancer cells. Cancer Gene Ther 11:109–120
Bautista DS, Xuan JW, Hota C, Chambers AF, Harris JF (1994) Inhibition of Arg-Gly-Asp (RGD)-mediated cell adhesion to osteopontin by a monoclonal antibody against osteopontin. J Biol Chem 269:23280–23285
Denhardt DT, Noda M (1998) Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl 30–31:92–102
Acknowledgements
The authors would like to thank Dr. Gwyn Bebb and Dr. Frank Jirik for the gift of cell lines. Thanks also to Elizabeth Kornaga for help with the statistical analysis and Ravinder Singh for careful reading of the manuscript. Grant support: Alberta Cancer Board, Breast Cancer Operating Grant Project #23141. Canadian Breast Cancer Foundation – Prairies/NWT Chapter, Project “Development of Diagnostic and Therapeutic Agents that recognize a Mutated Form of Src Kinase in Breast Cancer”. Canadian Institutes of Health Research Studentship support for K. Koro. Translational Research Training in Cancer Program Studentship for K. Koro. Alberta Heritage Foundation for Medical Research and Alberta Cancer Research Institute summer studentship support for K. Koro, S. Parkin.
Conflict of interest statement
None of the authors have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koro, K., Parkin, S., Pohorelic, B. et al. Interactions between breast cancer cells and bone marrow derived cells in vitro define a role for osteopontin in affecting breast cancer cell migration. Breast Cancer Res Treat 126, 73–83 (2011). https://doi.org/10.1007/s10549-010-0889-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0889-9