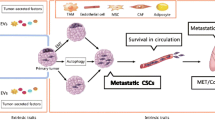
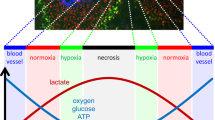
Abstract
The metastatic spread of tumor cells to distant sites represents the major cause of cancer-related deaths. Cancer metastasis involves a series of complex interactions between tumor cells and microenvironment that influence its biological effectiveness and facilitate tumor cell arrest to distant organs. More than a century since Paget developed the theory of seed and soil, the enigma of tissue specificity observed in metastatic colonization of tumor cells begins to unfold itself. The advent of new technologies has led to the discovery of novel molecules and pathways that confer metastasis-associated properties to the cancer cells, mediating organ specificity and unique genetic signatures have been developed using microarray studies. Future clinical studies and new antimetastatic compounds aiming to improve survival of patients with metastasis will most probably be based on these signatures. This review summarizes the plethora of old and new molecules that are strongly correlated with organ-specific metastases and which provide now an identity to the theory of seed and soil.
Similar content being viewed by others
References
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–0.
Fidler, I. J. (2002). Critical determinants of metastasis. Seminars Cancer Biology, 12, 89–6.
Fidler, I. J. (2002). The organ microenvironment and cancer metastasis. Differentiation, 70, 498–05.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet, 1, 571–73.
Sugarbaker, D. (1952). Organ selectivity of experimentally induced metastases in rats. Cancer, 5, 606–12.
Kinsey, D. L. (1960). An experimental study of preferential metastasis. Cancer, 13, 674–76.
Hart, I. R., & Fidler, I. J. (1980). Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Research, 40, 2281–287.
Loberg, R. D., Logothetis, C. J., Keller, E. T., & Pienta, K. J. (2005). Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. Journal of Clinical Oncology, 23, 8232–241.
Nielsen, O. S., Munro, A. J., & Tannock, I. F. (1991). Bone metastases: Pathophysiology and management policy. Journal of Clinical Oncology, 9, 509–24.
Guise, T. A., Mohammad, K. S., Clines, G., Stebbins, E. G., Wong, D. H., Higgins, L. S., et al. (2006). Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clinical Cancer Research, 12, 6213s–216s.
Roodman, G. (2004). Mechanisms of disease. Mechanisms of bone metastasis. New England Journal of Medicine, 350, 1655–664.
Mundy, G. (2002). Metastasis to the bone: Causes, consequences and therapeutic opportunities. Nature Reviews Cancer, 2, 584–93.
Logothetis, C. J., & Lin, S. H. (2005). Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer, 5, 21–8.
Koeneman, K. S., Yeung, F., & Chung, L. W. (1999). Osteomimetic properties of prostate cancer cells: A hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate, 39, 246–61.
Mundy, G. R. (2003). Endothelin-1 and osteoblastic metastasis. PNAS, 100, 10588–0589.
Shioide, M., & Noda, M. (2003). Endothelin modulates osteopontin and osteocalcin messenger ribonucleic acid expression in rat osteoblastic osteosarcoma cells. Journal of Cell Biochemistry, 53, 176–80.
Kozawa, O., Kawamura, H., Hatakeyama, D., Matsuno, H., & Uematsu, T. (2000). Endothelin-1 induces vascular endothelial growth factor synthesis in osteoblasts: Involvement of p38 mitogen-activated protein kinase. Cell Signal, 12, 375–80.
Nelson, J. B., Hedican, S. P., George, D. J., Reddi, A. H., Piantadosi, S., Eisenberger, M. A., et al. (1995). Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature Medicine, 1, 944–49.
Abe, E. (2006). Function of BMPs and BMP antagonists in adult bone. Annals of New York Academy of Sciences, 1068, 41–3.
Dai, J., Keller, J., Zhang, J., Lu, Y., Yao, Z., & Keller, E. T. (2005). Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Research, 65, 8274–285.
Asahina, I., Sampath, T. K., & Hauschka, P. V. (1996). Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Experimental Cell Research, 222, 38–7.
Honda, Y., Knutsen, R., Strong, D. D., Sampath, T. K., Baylink, D. J., & Mohan, S. (1997). Osteogenic protein-1 stimulates mRNA levels of BMP-6 and decreases mRNA levels of BMP-2 and -4 in human osteosarcoma cells. Calcified Tissue International, 60, 297–01.
Goya, M., Ishii, G., Miyamoto, S., Hasebe, T., Nagai, K., Yonou, H., et al. (2006). Prostate-specific antigen induces apoptosis of osteoclast precursors: Potential role in osteoblastic bone metastases of prostate cancer. Prostate, 66, 1573–584.
Nadiminty, N., Lou, W., Lee, S. O., Mehraein-Ghomi, F., Kirk, J. S., Conroy, J. M., et al. (2006). Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clinical Cancer Research, 12, 1420–430.
Clevers, H. (2006). Wt/beta-catenin signaling in development and disease. Cell, 127, 469–80.
Chen, G., Shukeir, N., Potti, A., Sircar, K., Aprikian, A., Goltzman, D., et al. (2004). Up-regulation of Wnt-1 and β-catenin production in patients with advanced metastatic prostate carcinoma. Cancer, 101, 1345–356.
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A., & Keller, E. T. (2005). Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Research, 65, 7554–560.
Shariat, S. F., Shalev, M., Menesses-Diaz, A., Kim, I. Y., Kattan, M. W., Wheeler, T. M., et al. (2001). Preoperative plasma levels of transforming growth factor β1 (TGF-β1) strongly predict progression in patients undergoing radical prostatectomy. Journal of Clinical Oncology, 19, 2856–864.
Cao, Y., Zhou, Z., de Crombrugghe, B., Nakashima, K., Guan, H., Duan, X., et al. (2005). Osterix, a transcription factor for osteoblast differentiation, mediates antitumor activity in murine osteosarcoma. Cancer Research, 65, 1124–128.
Nakashima, K., Zhou, X., Kunkel, G., Zhang, Z., Deng, J. M., Behringer, R. R., et al. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell, 108, 17–9.
Pratap, J., Lian, J. B., Javed, A., Barnes, G. L., van Wijnen, A. J., Stein, J. L., et al. (2006). Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer and Metastasis Reviews, 25, 589–00.
Barnes, G. L., Javed, A., Waller, S. M., Kamal, M. H., Hebert, K. E., Hassan, M. Q., et al. (2003). Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Research, 63, 2631–637.
Barnes, G. L., Hebert, K. E., Kamal, M., Javed, A., Einhorn, T. A., Lian, J. B., et al. (2004). Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Research, 64, 4506–513.
Sun, Y. X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J., et al. (2005). Skeletal localization and neutralization of the SDF-1/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research, 20, 318–29.
Vaday, G. G. (2004). CXCR4 and CXCL12 (SDF-1) in prostate cancer: Inhibitory effects of human single chain Fv antibodies. Clinical Cancer Research, 10, 5630–639.
Blair, J. M., Zhou, H., Seibel, M. J., & Dunstan, C. R. (2006). Mechanisms of disease: Roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nature Clinical Practice Oncology, 3, 41–9.
Dougall, W. C., & Chaisson, M. (2006). The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer and Metastasis Reviews, 25, 541–49.
Liao, J., & McCauley, L. K. (2006). Skeletal metastasis: Established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer and Metastasis Reviews, 25, 559–71.
Zhang, J., Dai, J., Yao, Z., Lu, Y., Dougall, W., & Keller, E. T. (2003). Soluble receptor activator of nuclear factor kB Fc diminishes prostate cancer progression in bone. Cancer Research, 63, 7883–890.
Neville-Webbe, H. L., Cross, N. A., Eaton, C. L., Nyambo, R., Evans, C. A., Coleman, R. E., et al. (2004). Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Research and Treatment, 86, 269–79.
Zhang, J., Dai, J., Qi, Y., Lin, D. L., Smith, P., Strayhorn, C., et al. (2001). Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. Journal of Clinical Investigation, 107, 1235–244.
Morony, S., Capparelli, C., Sarosi, I., Lacey, D. L., Dunstan, C. R., & Kostenuik, P. J. (2001). Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Research, 61, 4432–436.
Moussad, E. E., & Brigstock, D. R. (2002). Connective tissue growth factor: What’s in a name? Molecular Genetics and Metabolism, 71, 276–92.
Morgan, H., Tumber, A., & Hill, P. A. (2004). Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. International Journal of Cancer, 109, 653–60.
de Winter, J. P., ten Dijke, P., de Vries, C. J., van Achterberg, T. A., Sugino, H., de Waele, P., et al. (1996). Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Molecular Cell Endocrinology, 116, 105–14.
Leto, G., Incorvaia, L., Badalamenti, G., Tumminello, F. M., Gebbia, N., Flandina, C., et al. (2006). Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clinical & Experimental Metastasis, 23, 117–22.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2, 161–74.
Liu, Y. J., Xu, Y., & Yu, Q. (2006). Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene, 25, 2452–467.
Yang, Y., Macleod, V., Bendre, M., Huang, Y., Theus, A. M., Miao, H. Q., et al. (2005). Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood, 105, 1303–309.
Kang, S. H., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C., et al. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 3, 537–49.
Mukherji, S. K., Armao, D., & Joshi, V. M. (2001). Cervical nodal metastases in squamous cell carcinoma of the head and neck: What to expect. Head and Neck, 23, 995–005.
O’Donnell, R. K., Kupferman, M., Wei, S. J., Singhal, S., Weber, R., O’Malley, B., et al. (2005). Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene, 24, 1244–251.
Greenberg, J. S., Fowler, R., Gomez, J., Mo, V., Roberts, D., El Naggar, A. K., et al. (2004). Extent of extracapsular spread: A critical prognosticator in oral tongue cancer. Cancer, 97, 1464–470.
Zhou, X., Temam, S., Oh, M., Pungpravat, N., Huang, B. L., Mao, L., et al. (2006). Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia, 8, 925–32.
Roepman, P., Wessels, L. F., Kettelarij, N., Kemmeren, P., Miles, A. J., Lijnzaad, P., et al. (2005). An expression profile for diagnosis of lymph node metastases from primary head and neck squamous cell carcinomas. Nature Genetics, 37, 182–86.
Roepman, P., Kemmeren, P., Wessels, L. F., Slootweg, P. J., & Holstege, F. C. (2006). Multiple robust signatures for detecting lymph node metastasis in head and neck cancer. Cancer Research, 66, 2361–366.
Roepman, P., de Jager, A., Groot Koerkamp, M. J., Kummer, J. A., Slootweg, P. J., & Holstege, F. C. (2006). Maintenance of head and neck tumor gene expression profiles upon lymph node metastasis. Cancer Research, 66, 11110–1114.
Roepman, P., de Koning, E., van Leenen, D., de Weger, R. A., Kummer, J. A., Slootweg, P. J., et al. (2007). Dissection of a metastatic gene expression signature into distinct components. Genome Biology, 7, R117.
Chu, J. H., Sun, Z. Y., Meng, X. L., Wu, J. H., He, G. L., Liu, G. M., et al. (2006). Differential metastasis-associated gene analysis of prostate carcinoma cells derived from primary tumor and spontaneous lymphatic metastasis in nude mice with orthotopic implantation of PC-3M cells. Cancer Letters, 233, 79–8.
Kikuchi, T., Daigo, Y., Katagiri, T., Tsunoda, T., Okada, K., Kakiuchi, S., et al. (2003). Expression profiles of non-small cell lung cancers on cDNA microarrays: Identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene, 22, 2192–205.
Kobayashi, K., Imai, K., & Nakamura, Y. (2003). Expression profiles of non-small cell lung cancers on DNA microarrays: Identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene, 22, 2105–192.
Langer, C. J., & Mehta, M. P. (2005). Current management of brain metastases, with a focus on systemic options. Journal of Clinical Oncology, 23, 6207–219.
Kim, L. S., Huang, S., Lu, W., Lev, D. C., & Price, J. E. (2004). Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clinical & Experimental Metastasis, 21, 107–18.
Yano, S., Shinohara, H., Herbst, R. S., Kuniyasu, H., Bucana, C. D., Ellis, L. M., et al. (2000). Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Research, 60, 4959–967.
Sierra, A., Price, J., Garcia-Ramirez, M., Mendez, O., Lopez, L., & Fabra, A. (1997). Astrocyte derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Laboratory Investigation, 77, 357–68.
Entschladen, F., Drell, T. L., 4th, Lang, K., Joseph, J., & Zaenker, K. S. (2004). Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncology, 5, 254–58.
Drell, T. L., 4th, Joseph, J., Lang, K., Niggemann, B., Zaenker, K. S., & Entschladen, F. (2003). Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Research and Treatment, 80, 63–0.
Steeg, P. (2003). Metastasis suppressors alter the signal transduction of cancer cells. Nature Reviews Cancer, 3, 55–3.
Steeg, P. (2004). Perspectives on classic articles: Metastasis suppressor genes. Journal of the National Cancer Institute, 96, E4.
Stark, A. M., Tongers, K., Maass, N., Mehdorn, H. M., & Held-Feindt, J. (2005). Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. Journal of Cancer Research and Clinical Oncology, 31, 191.
Fidler, I. J. (1973). Selection of successive tumor lines for metastasis. Nature, 242, 148–49.
Fidler, I. J., Gruys, E., Cifone, M. A., Barnes, Z., & Bucana, C. (1981). Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. Journal of the National Cancer Institute, 67, 947–56.
Boukerche, H., Baril, P., Tabone, E., Berard, F., Sanhadji, K., Balme, B., et al. (2000). A new Mr 55,000 surface protein implicated in melanoma progression: Association with a metastatic phenotype. Cancer Research, 60, 5848–856.
Huang, S. (2007). Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: Clinical implications. Cancer Research, 13, 1362–366.
Xie, T. X., Huang, F. J., Aldape, K. D., Kang, S. H., Liu, M., Gershenwald, J. E., et al. (2006). Activation of stat3 in human melanoma promotes brain metastasis. Clinical Cancer Research, 66, 3188–196.
Lee, J. H., Miele, M. E., Hicks, D. J., Phillips, K. K., Trent, J. M, Weissman, B. E., et al. (1998). KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. Journal of the National Cancer Institute, 88, 1731–737.
Sarris, M., Scolyer, R. A., Konopka, M., Thompson, J. F., Harper, C. G., & Lee, C. S. (2004). Cytoplasmic expression of nm23 predicts the potential for cerebral metastasis in patients with primary cutaneous melanoma. Melanoma Research, 14, 23–7.
Denkins, Y., Reiland, J., Roy, M., Sinnappah-Kang, N. D., Galjour, J., Murry, B. P., et al. (2004). Brain metastases in melanoma: Roles of neurotrophins. Neuro-Oncology, 6, 154–65.
Marchetti, D., McQuillan, D. J., Spohn, W. C., Carson, D. D., & Nicolson, G. L. (1996). Neurotrophin stimulation of human melanoma cell invasion: Selected enhancement of heparanase activity and heparanase degradation of specific heparan sulfate subpopulations. Cancer Research, 56, 2856–863.
Marchetti, D., Li, J., & Shen, R. (2000). Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Research, 60, 4767–770.
Lin, U. N., & Winer, P. E. (2007). Brain metastases: The HER2 paradigm. Clinical Cancer Research, 13, 1648–655.
Kirsch, D. G., Ledezma, C. J., Mathews, C. S., Bhan, A. K., Ancukiewicz, M., Hochberg, F. H., et al. (2005). Survival after brain metastases from breast cancer in the trastuzumab era. Journal of Clinical Oncology, 23, 2114–116.
Palmieri, D., Bronder, J. L., Herring, J. M., Yoneda, T., Weil, R. J., Stark, A. M., et al. (2007). Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Research, 67, 4190–198.
Ohtaki, T., Shintani, Y., Honda, S., Matsumoto, H., Hori, A., Kanehashi, K., et al. (2001). Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature, 411, 613–17.
Lee, J. H., & Welch, D. R. (1997). Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Research, 57, 2384–387.
Bandyopadhyay, S., Zhan, R., Chaudhuri, A., Watabe, M., Pai, S. K., Hirota, S., et al. (2006). Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nature Medicine, 12, 933–38.
van der Horst, E. H., Degenhardt, Y. Y., Strelow, A., Slavin, A., Chinn, L., Orf, J., et al. (2005). Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proceedings of National Academy of Sciences of the United States of America, 102, 15901–5906.
Goodison, S., Yuan, J., Sloan, D., Kim, R., Li, C., Popescu, N. C., et al. (2005). The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Research, 65, 6042–053.
Zhang, T., Sun, H. C., Xu, Y., Zhang, K. Z., Wang, L., Qin, L. X., et al. (2005). Overexpression of platelet-derived growth factor receptor-α in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clinical Cancer Research, 11, 8557–563.
Brown, D., & Ruoslahti, E. (2004). Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell, 5, 365–74.
Khanna, C., Wan, X., Bose, S., Cassaday, R., Olomu, O., Mendoza, A., et al. (2004). The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature Medicine, 10, 182–86.
Minn, A. J., Gupta, G. P., Siegel, P. M., Bos, P. D., Shu, W., Giri, D. D., et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature, 436, 518–24.
Zhang, T., Sun, H. C., Xu, X., Zhang, K. Z., Wang, L., Qin, L. X., et al. (2005). Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clinical Cancer Research, 11, 8557–563.
Hwang, R. F., Yokoi, K., Bucana, C. D., Tsan, R., Killion, J. J., Evans, D. B., et al. (2003). Inhibition of platelet-derived growth factor receptor phosphorylation by STI571 (Gleevec) reduces growth and metastasis of human pancreatic carcinoma in an orthotopic nude mouse model. Clinical Cancer Research, 15, 6534–544.
Lee, J. H., Park, S. R., & Cha, K. O. (2004). KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the Tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Research, 64, 4235–243.
Saha, S., Bardelli, A., Buckhaults, P., Velculescu, V. E., Rago, C., St Croix, B., et al. (2001). A phosphatase associated with metastasis of colorectal cancer. Science, 294, 1343–346.
Kato, H., Semba, S., Miskad, U. A., Seo, Y., Kasuga, M., & Yokozaki, H. (2004). High expression of PRL-3 promotes cancer cell motility and liver metastasis in human colorectal cancer: A predictive molecular marker of metachronous liver and lung metastases. Clinical Cancer Research, 10, 7318–328.
Zeng, Q., Dong, J. M., Guo, K., Li, J., Tan, H. X., Koh, V., et al. (2003). PRL-3 and PRL-1 promote cell migration, invasion, and metastasis. Cancer Research, 63, 2716–722.
Fiordalisi, J. J., Keller, P. J., & Cox, A. D. (2006). PRL tyrosine phosphatases regulate Rho family GTPases to promote invasion and motility. Cancer Research, 66, 3153–158.
Li, J., Guo, K., Koh, V. W., Tang, J. P., Gan, B. Q., Shi, H., et al. (2005). Generation of PRL-3- and PRL-1-specific monoclonal antibodies as potential diagnostic markers for cancer metastases. Clinical Cancer Research, 11, 2104–195.
Miyamoto, S., Nakamura, M., Shitara, K., Nakamura, K., Ohki, Y., Ishii, G., et al. (2005). Blockade of paracrine supply of insulin-like growth factors using neutralizing antibodies suppresses the liver metastasis of human colorectal cancers. Clinical Cancer Research, 11, 3494–502.
Kabbinavar, F. F., Schulz, J., McCleod, M., Patel, T., Hamm, J. T., Hecht, J. R., et al. (2005). Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. Journal of Clinical Oncology, 23, 3697–705.
Yoo, P. S., Lopez-Soler, R. I., Longo, W. E., & Cha, C. H. (2006). Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clinical Colorectal Cancer, 6, 202–07.
Kindler, H. L., Friberg, G., Singh, D. A., Locker, G., Nattam, S., Kozloff, M., et al. (2005). Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. Journal of Clinical Oncology, 23, 8033–040.
Ellis, L. M., Curley, S. A., & Grothey, A. (2005). Surgical resection after downsizing of colorectal liver metastasis in the era of bevacizumab. Journal of Clinical Oncology, 23, 4853–855.
Yezhelyev, M. V., Koehl, G., Guba, M., Brabletz, T., Jauch, K. W., Ryan, A., et al. (2004). Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clinical Cancer Research, 10, 8028–036.
Stephan, S., Datta, K., Wang, E., Li, J., Brekken, R. A., Parangi, S., et al. (2004). Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clinical Cancer Research, 10, 6993–000.
Yuki, K., Hirohashi, S., Sakamoto, M., Kanai, T., & Shimosato, Y. (1990). Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer, 66, 2174–179.
Ye, Q. H., Qin, L. X., Forgues, M., He, P., Kim, J. W., Peng, A. C., et al. (2003). Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nature Medicine, 4, 416–23.
van ’t Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415, 530–36.
Budhu, A., Forgues, M., Ye, Q. H., Jia, H. L., He, P., Zanetti, K. A., et al. (2006). Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell, 10, 99–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fokas, E., Engenhart-Cabillic, R., Daniilidis, K. et al. Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 26, 705–715 (2007). https://doi.org/10.1007/s10555-007-9088-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-007-9088-5