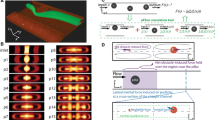
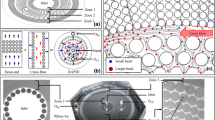
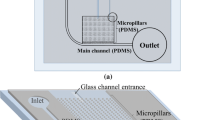
Abstract
Pillar-based passive microfluidic devices combine the advantages of simple designs, small device footprint, and high selectivity for size-based separation of blood cells. Most of these device designs have been validated with dilute blood samples. Handling whole blood in pillar-based devices is extremely challenging due to clogging. The high proportion of cells (particularly red blood cells) in blood, the varying sizes and stiffness of the different blood cells, and the tendency of the cells to aggregate lead to clogging of the pillars within a short period. We recently reported a ra dial pi llar d evice (RAPID) design for continuous and high throughput separation of multi-sized rigid polystyrene particles in a single experiment. In the current manuscript, we have given detailed guidelines to modify the design of RAPID for any application with deformable objects (e.g. cells). We have adapted RAPID to work with whole blood without any pre-processing steps. We were successful in operating the device with whole blood for almost 6 h, which is difficult to achieve with most pillar-based devices. The availability of multiple parallel paths for the cells and the provision for a self-generating cross flow in the device design were the main reasons behind the minimal clogging in our device. We also observed that a vibrator motor attached to the inlet tubing occasionally disturbed the cell clumps. As an illustration of the improved device design, we demonstrated up to ∼ 60-fold enrichment of platelets.






Similar content being viewed by others
References
X. Chen, C.C. Liu, H. Li, et al., Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B. 130(1), 216 (2008)
Y. Cheng, X. Ye, Z. Ma, S. Xie, W. Wang, High-throughput and clogging-free microfluidic filtration platform for on-chip cell separation from undiluted whole blood. Biomicrofluidics. 10(1), 014118 (2016)
S. Choi, T. Ku, S. Song, C. Choi, J.K. Park, Hydrophoretic high-throughput selection of platelets in physiological shear-stress range. Lab. Chip. 11(3), 413 (2011)
K.H. Chung, Y.H. Choi, J.H. Yang, C.W. Park, W.J. Kim, C.S. Ah, G.Y. Sung, Magnetically-actuated blood filter unit attachable to pre-made biochips. Lab. Chip. 12(18), 3272 (2012)
J.A. Davis, D.W. Inglis, K.J. Morton, D.A. Lawrence, L.R. Huang, S.Y. Chou, J.C. Sturm, R.H. Austin, Deterministic hydrodynamics: Taking blood apart. Proc. Natl. Acad. Sci. 103(40), 14779 (2006)
I.K. Dimov, L. Basabe-Desmonts, J.L. Garcia-Cordero, B.M. Ross, A.J. Ricco, L.P. Lee, Stand-alone self-powered integrated microfluidic blood analysis system (simbas). Lab. Chip. 11(5), 845 (2011)
Z. Geng, Y. Ju, W. Wang, Z. Li, Continuous blood separation utilizing spiral filtration microchannel with gradually varied width and micro-pillar array. Sens. Actuators B. 180, 122 (2013)
D.W. Inglis, N. Herman, G. Vesey, Highly accurate deterministic lateral displacement device and its application to purification of fungal spores. Biomicrofluidics. 4(2), 024109 (2010)
H.M. Ji, V. Samper, Y. Chen, C.K. Heng, T.M. Lim, L. Yobas, Silicon-based microfilters for whole blood cell separation. Biomed. Microdev. 10(2), 251 (2008)
K. Kamei, K. Tajima, N.T. Huy, T. Kariu, et al., One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar. J. 3(1), 7 (2004)
M. Kersaudy-Kerhoas, E. Sollier, Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab. Chip. 13(17), 3323 (2013)
D.S. Lee, Y.H. Choi, Y.D. Han, H.C. Yoon, S. Shoji, M.Y. Jung, Construction of membrane sieves using stoichiometric and stress-reduced si 3 n 4/sio 2/si 3 n 4 multilayer films and their applications in blood plasma separation. ETRI J. 34(2), 226 (2012)
C. Li, C. Liu, Z. Xu, J. Li, Extraction of plasma from whole blood using a deposited microbead plug (dmbp) in a capillary-driven microfluidic device. Biomed. Microdev. 14(3), 565 (2012)
R. Mariella, Sample preparation: The weak link in microfluidics-based biodetection. Biomedical microdevices. 10(6), 777 (2008)
S.M. McFaul, B.K. Lin, H. Ma, Cell separation based on size and deformability using microfluidic funnel ratchets. Lab. chip. 12(13), 2369 (2012)
J. McGrath, M. Jimenez, H. Bridle, Deterministic lateral displacement for particle separation: A review. Lab. Chip. 14(21), 4139 (2014)
N. Mehendale, O. Sharma, C. D’Costa, D. Paul, A radial pillar device (rapid) for continuous and high-throughput separation of multi-sized particles. Biomed. Microdev. 20(1), 6 (2018)
J. Moorthy, D.J. Beebe, In situ fabricated porous filters for microsystems. Lab. Chip. 3(2), 62 (2003)
J. Nam, H. Lim, D. Kim, H. Jung, S. Shin, Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-newtonian fluid. Lab. Chip. 12(7), 1347 (2012)
E. Sollier, H. Rostaing, P. Pouteau, Y. Fouillet, J.L. Achard, Passive microfluidic devices for plasma extraction from whole human blood. Sens. Actuators B. 141(2), 617 (2009)
T. Tachi, N. Kaji, M. Tokeshi, Y. Baba, Simultaneous separation, metering, and dilution of plasma from human whole blood in a microfluidic system. Anal. Chem. 81(8), 3194 (2009)
S. Thorslund, O. Klett, F. Nikolajeff, K. Markides, J. Bergquist, A hybrid poly (dimethylsiloxane) microsystem for on-chip whole blood filtration optimized for steroid screening. Biomed. Microdev. 8(1), 73 (2006)
V. VanDelinder, A. Groisman, Separation of plasma from whole human blood in a continuous cross-flow in a molded microfluidic device. Anal. Chem. 78(11), 3765 (2006)
V. Vickerman, J. Blundo, S. Chung, R. Kamm, Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab. Chip. 8 (9), 1468 (2008)
D. Wlodkowic, J.M. Cooper, Tumors on chips: Oncology meets microfluidics. Curr. Opinion Chem. Biol. 14(5), 556 (2010)
Y. Yoon, S. Kim, J. Lee, J. Choi, R.K. Kim, S.J. Lee, O. Sul, S.B. Lee, Clogging-free microfluidics for continuous size-based separation of microparticles. Sci. Rep. 6, 26531 (2016)
K.K. Zeming, T. Salafi, C.H. Chen, Y. Zhang, Asymmetrical deterministic lateral displacement gaps for dual functions of enhanced separation and throughput of red blood cells. Sci. Rep. 6, 22934 (2016)
Acknowledgments
We acknowledge funding for cleanroom access from the Centre for Nanoelectronics Phase 2 project (MeiTY, Govt. of India). All lithography was performed in the cleanroom of Indian Institute of Technology Bombay’s nanofabrication facility. Plasma bonding was carried out in the M. Tech. teaching lab of the Biosciences and Bioengineering Department, IIT Bombay. We thank the LSM confocal microscope facility housed in the Department of Biosciences and Bioengineering for acquiring the images in Fig.6. We acknowledge financial (travel) support from the Wadhwani Research Centre for Bioengineering. Finally, we thank Kaushalya Foundation Medical Trust Hospital for technical support and discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehendale, N., Sharma, O., Pandey, S. et al. Clogging-free continuous operation with whole blood in a radial pillar device (RAPID). Biomed Microdevices 20, 75 (2018). https://doi.org/10.1007/s10544-018-0319-z
Published:
DOI: https://doi.org/10.1007/s10544-018-0319-z