Abstract
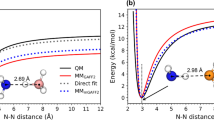
Comparative molecular dynamics simulations of a hexamer cluster of the protic ionic liquid ethylammonium nitrate are performed using density functional theory and density functional-based tight-binding (DFTB) methods. The focus is on assessing the performance of the DFTB approach to describe the dynamics and infrared spectroscopic signatures of hydrogen bonding between the ions. Average geometries and geometric correlations are found to be rather similar. The same holds true for the far-infrared spectral region. Differences are more pronounced for the NH- and CH-stretching bands, where DFTB predicts a broader intensity distribution. DFTB completely fails to describe the fingerprint range shaped by nitrate anion vibrations. Finally, charge fluctuations within the H bonds are characterized yielding moderate dependencies on geometry. On the basis of these results, DFTB is recommended for the simulation of H bond properties of this type of ionic liquid.







Similar content being viewed by others
References
Firaha DS, Hollóczki O, Kirchner B (2015) Angew Chem Int Ed 54:7805
Kerlé D, Ludwig R, Geiger A, Paschek D (2009) J Phys Chem B 113:12727
Welton T (1999) Chem Rev 99:2071
Zhang Y, Maginn EJ (2015) J Phys Chem Lett 6:700
Hunt PA, Ashworth CR, Matthews RP (2015) Chem Soc Rev 44:1257
Fumino K, Wulf A, Ludwig R (2008) Angew Chem Int Ed 47:8731
Fumino K, Reichert E, Wittler K, Hempelmann R, Ludwig R (2012) Angew Chem Int Ed 51:6236
Roth C, Chatzipapadopoulos S, Kerlé D, Friedriszik F, Lütgens M, Lochbrunner S, Kühn O, Ludwig R (2012) New J Phys 14:105026
Nibbering ET, Dreyer J, Kühn O, Bredenbeck J, Hamm P, Elsaesser T (2007) In: Kühn O, Wöste L (eds) Analysis and control of ultrafast photoinduced reactions. Springer, Heidelberg, p 619
Zhuang W, Hayashi T, Mukamel S (2009) Angew Chem Int Ed 48:3750
Chatzipapadopoulos S, Zentel T, Ludwig R, Lütgens M, Lochbrunner S, Kühn O (2015) ChemPhysChem 16:2519
Johnson CJ, Fournier JA, Wolke CT, Johnson MA (2013) J Chem Phys 139:224305
Bodo E, Sferrazza A, Caminiti R, Mangialardo S, Postorino P (2013) J Chem Phys 139:144309
Giese K, Petković M, Naundorf H, Kühn O (2006) Phys Rep 430:211
Mathias G, Baer MD (2011) J Chem Theory Comput 7:2028
Thomas M, Brehm M, Hollóczki O, Kelemen Z, Nyulászi L, Pasinszki T, Kirchner B (2014) J Chem Phys 141:024510
May V, Kühn O (2011) Charge and energy transfer dynamics in molecular systems, 3rd revised and enlarged edition. Wiley-VCH, Weinheim
Ivanov SD, Witt A, Marx D (2013) Phys Chem Chem Phys 15:10270
Maginn EJ (2009) J Phys Condens Matt 21:373101
Del Pópolo MG, Voth GA (2004) J Phys Chem B 108:1744
Bernardes CES, Shimizu K, Lobo Ferreira AIMC, Santos LMNBF, Canongia Lopes JN (2014) J Phys Chem B 118:6885
Shimizu K, Bernardes CES, Canongia JN (2014) J Phys Chem B 118:567
Zentel T, Kühn O (2016) J Chem Phys 145:234504
Marx D, Hutter J (2010) Ab initio molecular dynamics: basic theory and advanced methods. Cambridge University Press, Cambridge
Kirchner B, Hollóczki O, Canongia Lopes JN, Pádua AAH (2015) WIREs Comput Mol Sci 5:202
Zahn S, Thar J, Kirchner B (2010) J Chem Phys 132:124506
Schröder C (2011) J Chem Phys 135:024502
Wendler K, Brehm M, Malberg F, Kirchner B, Delle L (2012) J Chem Theory Comput 8:1570
Thomas M, Brehm M, Kirchner B (2015) Phys Chem Chem Phys 17:3207
Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim T, Suhai S, Seifert G (1998) Phys Rev B 58:7260
Yang Y, Yu H, York D, Cui Q, Elstner M (2007) J Phys Chem A 111:10861
Elstner M (2006) Theor Chem Acc 116:316
Welke K, Watanabe HC, Wolter T, Gaus M, Elstner M (2013) Phys Chem Chem Phys 15:6651
Addicoat MA, Stefanovic R, Webber GB, Atkin R, Page AJ (2014) J Chem Theory Comput 10:4633
Bodo E, Mangialardo S, Ramondo F, Ceccacci F, Postorino P (2012) J Phys Chem B 116:13878
Song X, Hamano H, Minofar B, Kanzaki R, Fujii K, Kameda Y, Kohara S, Watanabe M, Ishiguro Si, Umebayashi Y (2012) J Phys Chem B 116:2801
Fumino K, Wulf A, Ludwig R (2009) Angew Chem Int Ed 48:3184
Zentel T, Kühn O (2016) J Mol Liq 226:56
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Gottwald F (2012) Bachlor thesis. University of Rostock
Ufimtsev I, Martinez T (2009) J Chem Theory Comput 5:2619
Gaus M, Cui Q, Elstner M (2011) J Chem Theory Comput 7:931
Gaus M, Goez A, Elstner M (2013) J Chem Theory Comput 9:338
Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E (2001) J Chem Phys 114:5149
Aradi B, Hourahine B, Frauenheim T (2007) J Phys Chem A 111:5678
Brehm M, Weber H, Pensado AS, Stark A, Kirchner B (2012) Phys Chem Chem Phys 14:5030
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Chem Phys Lett 162:165
TURBOMOLE V6.5 (1989–2007) A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, TURBOMOLE GmbH, since 2007. http://www.turbomole.com
Deglmann P, May K, Furche F, Ahlrichs R (2004) Chem Phys Lett 384:103
Häser M, Ahlrichs R (1989) J Comput Chem 10:104
Hätting C, Weigend F (2000) J Chem Phys 113:5154
Hayes R, Imberti S, Warr GG, Atkin R (2011) Phys Chem Chem Phys 13:3237
Limbach HH, Denisov GS, Golubev NS (2006) In: Kohen A, Limbach HH (eds) Isotope effects in chemistry and biology. CRC Press, Boca Raton, p 193
Pauling L (1947) J Am Chem Soc 69:542
Limbach HH, Pietrzak M, Sharif S, Tolstoy PM, Shenderovich IG, Smirnov SN, Golubev NS, Denisov GS (2004) Chem Eur J 10:5195
Steiner T (1998) J Phys Chem A 102:7041
Munkres J (1957) J Soc Ind Appl Math 5:32
Otte N, Scholten M, Thiel W (2007) J Phys Chem A 111:5751
Umebayashi Y, Chung WL, Mitsugi T, Fukuda S, Takeuchi M, Fujii K, Takamuku T, Kanzaki R, Ishiguro Si (2008) J Comput Chem Jpn 7:125
Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG) for financial support through the SFB 652 and A. Wulf for supplying the experimental FIR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zentel, T., Kühn, O. Properties of hydrogen bonds in the protic ionic liquid ethylammonium nitrate. Theor Chem Acc 136, 87 (2017). https://doi.org/10.1007/s00214-017-2119-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-017-2119-6