Abstract
We describe a model for the Compound Action Currents (CACs) and Compound Action Potentials (CAPs) produced by a peripheral nerve bundlein vitro. The Single Fiber Action Currents (SFACs) and the extracellular Single Fiber Action Potentials (SFAPs) are calculated using a generalized volume conduction model. Frequency-dependent conductivities, variations in the intracellular action potentials with recording temperature and axon conduction velocity, and the effects of axonal myelination are incorporated into the volume conduction calculation. We demonstrate how the propagation distance and the recording radius affect the simulated Compound Action Signals (CASs) of various nerve bundles. We also demonstrate how the frequency-dependent and-independent conductivities affect the CASs simulated by our model. For this simulation, some of the parameters for the nerve bundles and Conduction Velocity Distributions (CVDs) were obtained from the literature. In accompanying papers, we use the simulated CASs to investigate the effects of variations in the model parameters on the CVDs predicted by our inverse model.
Similar content being viewed by others
Abbreviations
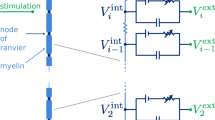
- φ i (ρ,z):
-
intracellular potential (mV)
- φ e (ρ,z):
-
potential in the saline bath (mV)
- φ s (ρ,θ,z):
-
sheath potential (mV)
- φ b (ρ,θ,z):
-
bundle potential (mV)
- φ m (z):
-
transmembrane potential (mV)
- φ i (ρ,k):
-
Fourier transform of the intracellular potential (mV·k)
- φ e (k):
-
Fourier transform of the potential in the saline bath (mV·k)
- φ m (k):
-
Fourier transform of the transmembrane potential (mV·k)
- k :
-
spatial frequency (1/mm)
- t :
-
time (ms)
- a :
-
axon radius (mm)
- p :
-
volume fraction
- ρ:
-
recording radius (mm)
- ν:
-
conduction velocity of the action potential (m/sec)
- σ i :
-
intracellular conductivity of the axon in the nerve bunde (Ω−1m−1)
- σ e :
-
extracellular conductivity of the saline bath (Ω−1m−1)
- σ s :
-
conductivity of the sheath around the nerve bundle (Ω−1m−1)
- σ z :
-
axial conductivity of the nerve bundle (Ω−1m−1)
- σ ρ :
-
radial conductivity of the nerve bundle (Ω−1m−1)
- b :
-
radius of the nerve bundle (mm)
- δ:
-
thickness of the sheath around the nerve bundle (mm)
- s :
-
distance of the axon from the center of the nerve bundle (mm)
- \(\bar s\) :
-
mean distance between center of bundle and center of axon (mm)
- A :
-
cross sectional area of the bundle (mm2)
- d * :
-
internal diameter of the nerve fiber (μm)
- τ j :
-
time delay between the stimulus time and the arrival time at the recording site of thejth nerve fiber (msec)
- D :
-
external diameter of the nerve fiber (μm)
- Z m :
-
membrane impedance times unit area (Ωm2)
- λ:
-
length constant (mm)
- B :
-
magnetic field (pT)
- c :
-
inner radius of the toroid (mm)
- d :
-
outer radius of the toroid (mm)
- e :
-
width of the toroid (mm)
- ρ eff :
-
effective toroid radius (mm)
- Γ:
-
circumference of the circle with radius ρ eff
- ω:
-
temporal angular frequency (1/s)
- T :
-
temperature (°C)
- l :
-
the propagation distance of the action signal (mm)
- N :
-
number of active fibers in the nerve bundle
- Υ:
-
delay of SFAS due to activation time and virtual cathode effect (msec)
- l n :
-
internodal distance (mm)
- Ј:
-
current density (A/m2)
- Iin :
-
total current passing through the toroid (A)
- L n :
-
length of the node of Ranvier (μm)
- C N :
-
capacitance of the node of Ranvier (F)
- C m :
-
effective membrane capacitance per unit area (F/m2)
- R N :
-
resistance of the node of Ranvier (Ω)
- R m :
-
effective resistance times unit area (Ωm2)
- R sh :
-
resistance of the sheath (Ω)
- C sh :
-
capacitance of the sheath (F)
- Q 10 :
-
temperature coefficient
- ATD:
-
arrival time distribution
- CAC:
-
compound action current (μA)
- CAP:
-
compound action potential (μV)
- CAS:
-
compound action signal (μA or μV)
- CVD:
-
conduction velocity distribution
- FDH:
-
fiber diameter histogram
- FFT:
-
fast Fourier transform
- NNLS:
-
nonnegative least squares
- SFAC:
-
single fiber action current (μA)
- SFAP:
-
single fiber action potential (μV)
- SFAS:
-
single fiber action signal (μA or μV)
References
Barach, J.P.; Freeman, J.A.; Wikswo, J.P. Experiments on the magnetic field of nerve action potentials. J. Appl. Physiol. 51:4532–4538; 1980.
Barach, J.P.; Roth, B.J.; Wikswo, J.P. Magnetic measurements of action currents in a single nerve axon: A core-conductor model. IEEE Trans. Biomed. Eng. BME-32 2:136–140; 1985.
Barker, A.T.; Brown, B.H.; Freeston, I.L. Modeling of an active nerve fiber in a finite volume conductor and its application to the calculation of surface action potentials. IEEE Trans. Biomed. Eng. BME-26:53–56; 1979a.
Barker, A.T.; Brown, B.H.; Freeston, I.L. Determination of the distribution of conduction velocities in human nerve trunks. IEEE Trans. Biomed. Eng. 26:76–81; 1979b.
Bishop, G.H.; Heinbecker, P. Differentiation of axon types of visceral nerves by means of the potential record. Amer. J. Physiol. 94:170–198; 1930.
Blair, E.A.; Erlanger, J. A comparison of the characteristics of axons through their individual electrical response. Amer. J. Physiol. 106:524–556; 1933.
Buchthal, F.; Rosenfalck, A. Evoked action potentials and conduction velocity in human sensory nerves. Brain Res. 3:1–122; 1966.
Buchthal, F.; Rosenfalck, A. Sensory potentials in polyneuropathy. Brain Res. 94:241–262; 1971.
Clark, J.W.; Plonsey, R. A mathematical evaluation of the core conduction model. Biophys. J. 6:95–112; 1966.
Clark, J.W.; Plonsey, R. The extracellular potential of the single active nerve fiber in a volume conductor. Biophys. J. 8:842–864; 1968.
Clark, J.W.; Plonsey, R. A mathematical study of nerve fiber interaction. Biophys. J. 8:842–864; 1968.
Cummins, K.L.; Perkel, D.H.; Dorfman, L.J. Nerve conduction velocity distributions. I. Estimation based on the single-fiber and compound action potentials. Electroenceph. Clin. Neurophysiol. 46:634–646; 1979a.
Cummins, K.L.; Dorfman, L.J.; Perkel, D.H. Nerve conduction velocity distributions. II. Estimation based on two compound action potentials. Electroenceph. Clin. Neurophysiol. 46:647–658; 1979b.
de Jesus, P.V.; Hausmanowa-Petrusewiez, I.; Barchi, R.L. The effect of cold on nerve conduction of human slow and fast nerve fibers. Neurology (Minneap). 23:1182–1189; 1973.
de Nó, R.L. A study of nerve physiology. Studies from the Rockefeller Inst. for Med. Res. 132:348–477; 1947.
Dyck, P.J.; Thomas, P.K.; Lambert, E.H.; Bunge, R. Peripheral neuropathy. Philadelphia: W.B. Saunders Company; 1984.
Erlanger, J.; Bishop, G.H.; Gasser, H.S. Experimental analysis of the simple action potential wave in nerve by the cathode ray oscillograph. Amer. J. Physiol. 78:537–573; 1926.
Erlanger, J.; Gasser, H.S. The action potential in fibers of slow conduction spinal roots and somatic nerves. Amer. J. Physiol. 92:43–59; 1930.
Erlanger, J.; Gasser, H.S. Electrical signs of nervous activity. London: Oxford Univ. Press; 1937.
Erlanger, J.; Gasser, H.S.; Bishop, G.H. The compound nature of the action current of nerve as disclosed by the cathode ray oscillograph. Amer. J. Physiol. 70:624–666; 1924.
Erne, S.N.; Curio, G.; Trahms, L.; Trontelj, Z.; Aust, P. Proc. 6th International Conference on Biomagnetism 166–169; 1987.
Gasser, H.S.; Erlanger, J. A study of the action currents of nerve with the cathode ray oscillograph. Amer. J. Physiol. 58:497–524; 1922a.
Gasser, H.S.; Erlanger, J. The components of action currents obtained from nerves. Amer. J. Physiol. 59:417–418; 1922b.
Gasser, H.S.; Erlanger, J. The role played by the sizes of the constituent fibers of the nerve trunk in determining the form of its action potential wave. Amer. J. Physiol. 80:522–545; 1927.
Gasser, H.S.; Grundfest, H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Amer. J. Physiol. 127:393–414; 1939.
Gielen, F.L.H.; Roth, B.J.; Wikswo, Jr., J.P. Capabilities of a toroid-amplifier system for magnetic measurement of current in biological tissue. IEEE Trans. Biomed. Eng. BME-33 10:910–921; 1986a.
Gielen, F.L.H.; Cruts, H.E.P.; Alberts, B.A.; Boon, K.L.; Wallinga de Jonge, W.; Boom, H.B.K. Model of electrical conductivity of skeletal muscle based on tissue structure. Med. and Biol. Eng. and Comput. 24:34–40; 1986b.
Gielen, F.L.H.; Roth, B.J.; Wikswo, J.P.; Brink, P. Action current propagation across an electric synapse: Magnetic measurements on a septated earthworm axon. (abst) Biophys. J. 49:340a; 1986c.
Helmholtz, H. Messungen uber den zeitlichenn Verlauf der Zuchung animalischer Muskeln und die Fortpflanzungsqeschwindigkeit der Reizung in den Nerven. Arch. Anat. Physiol. 276–364; 1850.
Heringa, A.; Stegeman, D.F.; Uijen, G.J.H.; De Weerd, J.P.C. Solution methods of electrical field problems in physiology. IEEE Trans. Biomed. Eng. BME-29:34–42; 1982.
Hutchinson, N.A.; Koles, Z.J.; Smith, R.S. Conduction velocity in myelinated nerve fibers ofXenopus Laevis. J. Physiol. 208:279–289; 1970.
Jackson, J. Classical electrodynamics. New York: Wiley; 1975.
Landau, W.M.; Clare, M.H.; Bishop, G.H. Reconstruction of myelinated nerve track action potentials: an arithmetic method. Exp. Neurol. 22:480–490; 1968.
Leifer, M.C.; Wikswo, Jr., J.P. Optimization of a clip-on SQUID current probe. Rev. Sci. Instrum. 54(8):1017–1022; 1983.
Moore, J.W.; Joyner, R.W.; Brill, M.N.; Waxman, M.H.; Najar-Ioa, M. Simulations of conduction in myelinated fibers: Relative sensitivity to changes in nodal and internodal parameters. Biophys. J. 21:147–160; 1978.
Olson, W.H.; BeMent, S.L. Compound action potential reconstructions and predicted fiber diameter distributions. In: Dorfman, L.J.; Cummins, K.L.; Leifer, L.J., eds. Conduction velocity distributions: A population approach to electrophysiology of nerve. New York: Alan R. Liss, Inc.; 1981.
Paintal, A.S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibers of the cat. J. Physiol. (Lond) 180:20–49; 1965.
Paintal, A.S. The influence of diameter of medullated nerve fibers of cats on the rising and falling phase of the spike and its recovery. J. Physiol. (Lond) 184:791–811; 1966.
Plonsey, R. Bioelectric phenomena. New York: McGraw-Hill; 1969.
Rosenfalck, P. Intra- and extracellular potential fields of active nerve and muscle fibers. Acta. Physiol. Scand. Suppl. 321:1–168; 1969.
Roth, B.J.; Wikswo, Jr., J.P. The electric potential and the magnetic field of an axon in a nerve bundle. Math. Biosci. 76:37–57; 1985a.
Roth, B.J.; Wikswo, Jr., J.P. The magnetic field of a single axon. Biophysical J. 48:93–109; 1985b.
Roth, B.J.; Gielen, F.L.H.; Wikswo, Jr., J.P. Spatial and temporal frequency dependent conductivities in volume conduction calculations of skeletal muscle. Math. Biosci. 88:159–189; 1988.
Rushton, W.A.H. A theory of the effects of fibre size in medullated nerve. J. Physiol. (Lond) 115:101–122; 1951.
Schoonhoven, R.; Stegeman, D.F.; De Weerd, J.P.C. The forward problem in electroneurography I: A generalized volume conduction model. IEEE Trans. Biomed. Eng. BME-33:327–334; 1986.
Schoonhoven, R.; Stegeman, D.F.; Oosterom, A.V.; Dautzenberg, G.F.M. The inverse problem in electroneurography I: Conceptual basis and mathematical formulation. IEEE Trans. Biomed. Eng. BME-35:769–777; 1988.
Stampfli, R. In: Waxman, S.G.; Ritchie, J.M., eds. Demyelinating disease, Basic clinical electrophysiology. New York: Raven; 1981.
Stegeman, D.F.; De Weerd, J.P.C.; Eijkman, E.G.J. A volume conductor study of compound action potential study of nervesin situ: The forward problem. Biol. Cybern. 33:97–111; 1979.
Stegeman, D.F.; De Weerd, J.P.C. Modelling compound action potentials of peripheral nervesin situ. I Model description; evidence for a non-linear relation between fibre diameter and velocity. Electroenceph. Clin. Neurophysiol. 54:436–448; 1982.
Swinney, K.; Wikswo, J.P. A calculation of the magnetic field of a nerve action potential. Biophys. J. 32:719–732; 1980.
Tasaki, I.; Frank, K. Measurement of the action potential of myelinated nerve fibre. Amer. J. Physiol. 182:572–578; 1955.
Tasaki, I.; Fujita, M. Action current of single nerve fibers as modified by temperature changes. J. Neurophysiol. 182:311–315; 1948.
Weerasuriya, A.; Spangler, R.; Rapoport, S.; Taylor, R. AC impedance of the perineurium of the frog sciatic nerve. Biophys. J. 46:167–174; 1984.
Wiederholt, W.C. Threshold and conduction velocity in isolated mixed mammalian nerves. Neurology 20:347–352; 1970.
Wijesinghe, R.S. Comparison of electric and magnetic techniques for the determination of conduction velocity distributions of nerve bundles. Vanderbilt Univ.; 1988. PhD Thesis.
Wijesinghe, R.S.; Wikswo, Jr., J.P. A model for compound action potentials and currents in a nerve bundle II: A sensitivity analysis of model parameters for the forward and inverse calculations. Ann. Biomed. Eng. 19:73–96.
Wijesinghe, R.S.; Gielen, F.L.H.; Wikswo, Jr., J.P. A model for compound action potentials and currents in a nerve bundle III: A comparison of the conduction velocity distributions calculated from compound action currents and potentials. Ann. Biomed. Eng. 19:97–121.
Wikswo, Jr. J.P.; Barach, J.P.; Freeman, J.A. Magnetic field of a nerve impulse: First measurements. Science 208:53–55; 1980.
Wikswo, Jr. J.P.; Barach, J.P.; Gunderson, S.C.; Palmer, J.O.; Freeman, J.A. Magnetic measurement of action currents in an isolated lobster axon. Il Nuovo Cimento 2D:368–378; 1983a.
Wikswo, Jr., J.P.; Samson, P.C.; Giffard, R.P. A low-noise low input impedance amplifier for magnetic measurements of nerve action currents. IEEE Trans. Biomed. Eng. BME-30:215–221; 1983b.
Wikswo, Jr., J.P.; Abraham, G.S.; Hentz, V.R. Magnetic assessment of regeneration across a nerve graft. In: Weinberg, H.; Stronik, G.; Katila, K., eds. Biomagnetism application and theory. New York: Pergamon Press; 1985a.
Wikswo, Jr., J.P.; Henry, W.P.; Samson, P.C.; Giffard, R.P. A current probe for measuring cellular action currents. In: Weinberg, H.; Stronik, G.; Katila, K., eds. Biomagnetism application and theory. New York: Pergamon Press; 1985b.
Wikswo, Jr., J.P.; Friedman, R.N.; Kilroy, A.W.; van Egeraat, J.M.; Buchanan, D.S. Preliminary measurements with MicroSQUID. In: Williamson, S.J., ed. Advances in biomagnetism. New York: Pergamon Press, 681–684; 1989.
Sepulveda, N.G.; Roth, B.J.; Wikswo, Jr., J.P. Current injection into a two-dimensional anisotropic bidomain. Biophys. J. 55:987–999; 1989.
Wilson, O.W.; Clark, J.W.; Ganapathy, N.; Harman, T.L. Potential field from an active nerve in an inhomogeneous, anisotropic volume conductor—the forward problem. IEEE Trans. Biomed. Eng. BME-32:1032–1041; 1985.
Woosley, J.K.; Roth, B.J.; Wikswo, Jr., J.P. The magnetic field of a single axon: A volume conductor model. Math. Biosci. 76:1–36; 1985.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wijesinghe, R.S., Gielen, F.L.H. & Wikswo, J.P. A model for compound action potentials and currents in a nerve bundle I: The forward calculation. Ann Biomed Eng 19, 43–72 (1991). https://doi.org/10.1007/BF02368460
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02368460