Abstract
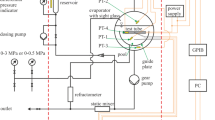
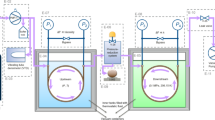
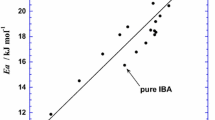
The viscosity and thermal conductivity of acetic acid water mixtures were measured over the entire composition range and at temperatures ranging from 293 to 453 K. Viscosity measurements were performed with a high-pressure viscometer and thermal conductivity was measured using a modified transient hot-wire technique. A mercury filled. glass capillary was used as the insulated hot wire in the measurements. The l iscosity data showed unusual trends with respect to composition. At it given temperature. the viscosity was seen to increase with increasing acid concentration, attain a maximum. and then decrease. The thermal conductivity, on the other hand, decreased monotonically with acid concentration. A generalized corresponding-states principle using water and acetic acid as the reference fluids was used to predict both viscosity and thermal conductivity with considerable sucres.
Similar content being viewed by others
References
E. R. Peterson.Measurement ol Thermal Conductivity of Aqueous Organic Liquids Using the Relative Transient Hot-Wi re Technique. Ph.D. thesis (Oklahoma State University, 1994).
I. U. Usmanov,Izvest. Acad. Nauk Uzbek. SSR Ser. Tekh. Nauk 6:61 (1968).
T. F. Sun. D. Ly, and A. S. Teja,I & EC Res. c39:1327 (1995).
R. J. Lee, R. M. DiGuilio. S. M. Jeter. and A. S. Teja,ASHRAE Trans. 96:723 (1990).
J. A. Riddick. W. B. Bunger, and T. K. Sakano (eds.).Organic Solvents. Physical Properties and Methods ol Purification, 4th ed. (Wiley New York, 1986). pp. 74–75.
R. M. DiGuilio, R. J. Lee. S. M. Jeter, and A. S. Teja.ASHRAE Trans. 96:702 (1990).
J. G. Bleazard. R. M. DiGuilio, and A. S. Teja.AIChE Symp. Ser. 298 90:23 (1994).
J. G. Bleazard and A. S. Teja.J. Chem. Eng. Data 40:732 (1995).
H. S. Carslaw and J. C. Jaegar.Conduction of Heat in Solids. 2nd ed. (Oxford University Press, London. 1959). pp. 188–213.
J. J. Healy, J. J. de Groot, and J. Kestin,Physica 82C:392 (1976).
M. L. V. Ramires. J. M. N. A. Fareleira. C. A. Nieto de Castro, M. Dix, and W. A. Wakeham.Int. J. Thermophys. 14:1119 (1993).
K. N. Marsh (ed.).Recommended Reference Materials for the Realization of Physiochemical Properties (Blackwell, Boston, 1987). p. 344.
W. M. Melzer. W. Baldauf. and H. Knapp,Chem. Eng. Process. 26:71 (1989).
A. S. Teja, S. I. Sandler, and N. C. Patel,Chem. Eng. J. 21:21 (1981).
A. S. Teja and P. Rice,I & EC Fund. 20:77 (1980).
A. S. Teja and P. Rice,Chem. Eng. Sri. 36:7 (1981).
A. S. Teja and P. Rice.Chem. Eng. Sri. 36:417 1981
A. S. Teja and P. Rice,Chem. Eng. Sci. 37:788 (1982)
T. E. Daubert and R. P. Damner (eds.),Dala Compilation Tables u/ Properties o/ Pure Compounds (Design Institute for Physical Property Data, American Institute of Chemical Engineers. New York, (1985).
J. Kestin and J. H. Whitelaw,Trans. ASME 88:82 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bleazard, J.G., Sun, T.F. & Teja, A.S. The thermal conductivity and viscosity of acetic acid-water mixtures. Int J Thermophys 17, 111–125 (1996). https://doi.org/10.1007/BF01448214
Issue Date:
DOI: https://doi.org/10.1007/BF01448214