Abstract
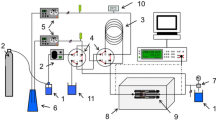
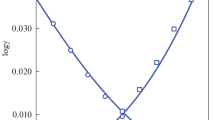
The D/H ratios of hydrogen gas in equilibrium with aqueous sodium chloride solutions of 2, 4 and 6 molalities were determined within the range 10 to 95°C, using a hydrophobic platinum catalyst. With each of the different sodium chloride concentrations, the hydrogen isotope effect between the solution and pure water changes linearly with the square of the reciprocal temperature. On the basis of the results for hydrogen isotope fractionation observed in this study, and those of hydrogen isotope fractionation between pure water and vapor, it is concluded that the structure of the aqueous sodium chloride solution does not change significantly with temperature. The hydrogen isotope effect is evidently different from the results of vapor pressure isotope effects (VPIE) on sodium chloride solutions measured on separated isotopes. The difference between the present work and the VPIE studies is probably due to a non-ideal behavior in a mixture of isotopic water molecules and/or to a H2O-D2O disproportionation reaction in sodium chloride solutions. The distinction between the latter two mechanisms can not be differentiated at present.
Similar content being viewed by others
References
H. Taube,J. Phys. Chem. 58, 523 (1954).
A. H. Truesdell,Earth Planet Sci. Lett. 23, 387 (1974).
M. K. Stewart and I. Friedman,J. Geophys. Res. 80, 3812 (1975).
M. Kakiuchi and S. Matsuo,J. Phys. Chem. 89, 4627 (1985).
H. M. Feder and H. Taube,J. Chem. Phys. 20, 1335 (1952).
Z. Sofer and J. R. Gat,Earth Planet Sci. Lett. 15, 232 (1972).
R. H. Betts, J. Bron, W. D. Buchannon, and K. D. Wu,Can. J. Chem. 55, 2966 (1977).
P. Bopp, K. Heinzinger, and A. Klemm,Z. Naturforsch. 32a, 1419 (1977).
J. R. O'Neil and A. H. Truesdell, inStable Isotope Geochemistry: A Tribute to Samuel Epstein, No. 3, (Geochemical Soceity Special Publ., 1991) p. 17.
Z. Sofer and J. R. Gat,Earth Planet Sci. Lett. 26, 179 (1975).
M. Kakiuchi,Z. Naturforsch. 43a, 449 (1988).
C. M. Graham and S. M. F. Sheppard,Earth Planet Sci. Lett. 49, 237 (1980).
K. Kazahaya, Ph.D. Thesis, Tokyo Institute of Technology (1986), Chap. II.
J. Horita, D. J. Wesolowski, and D. R. Cole,Geochim. Cosmochim. Acta 57, 2797 (1993).
J. H. Rolston, J. den Hartog, and J. P. Butler,J. Phys. Chem. 80, 1064 (1976).
H. Craig,Geochim. Cosmochim. Acta 12, 133 (1957).
S. Epstein and T. Mayeda,Geochim. Cosmochim. Acta 4, 213 (1953).
H. C. Urey,J. Chem. Soc. (London) 562 (1947).
J. Bigeleisen and M. G. Mayer,J. Chem. Phys. 15, 261 (1947).
M. Majoube,J. Chim. Phys. 68, 1423 (1971).
R. E. Criss, inStable Isotope Geochemistry: A Tribute to Samuel Epstein, No. 3, (Geochemical Soceity Special Publ., 1991) p. 11.
L. Merlivat, R. Botter, and G. Nief,J. Chim. Phys. 60, 56 (1963).
Y. Bottinga, Ph.D.Thesis, University of California (1968), Chap. V.
G. D. Oliver and J. W. Grisard,J. Am. Chem. Soc. 78, 561 (1956).
R. L. Combs and H. A. Smith,J. Phys. Chem. 61, 441 (1957).
C. T. Liu and W. T. Lindsay,J. Chem. Eng. Data 15, 510 (1970).
J. Pupezin, G. Jakli, G. Jancso, and W. A. Van Hook,J. Phys. Chem. 76, 743 (1972).
M. Kakiuchi and S. Matsuo,Geochem. J. 13, 307 (1979).
W. M. Jones,J. Chem. Phys. 48, 207 (1968).
G. Jakli and W. A. Van Hook,J. Chem. Eng. Data 26, 243 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kakiuchi, M. Temperature dependence of fractionation of hydrogen isotopes in aqueous sodium chloride solutions. J Solution Chem 23, 1073–1087 (1994). https://doi.org/10.1007/BF00976257
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00976257