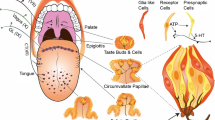
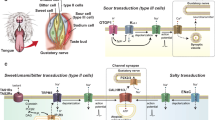
Abstract
Previously, we reported that agonists for the calcium-sensing receptor CaSR enhance the intensity of salty, sweet, and umami tastes. Interestingly, CaSR agonists enhance specific basic tastes but do not elicit any taste themselves. In the present study, we characterized how CaSR agonists specifically affect sweet taste. We found that the CaSR agonist, γ-glutamyl-valinyl-glycine, enhanced ATP secretion from isolated mouse circumvallate taste buds triggered by an artificial sweetener, SC45647. This enhancement was abolished by atropine, implicating the involvement of muscarinic cholinergic mechanisms in the perception of kokumi substances. Moreover, nearly all CaSR-immunopositive taste cells co-expressed cholinergic markers, suggesting that CaSR-positive taste cells contain acetylcholine. These observations indicate that CaSR agonists enhance sweet-induced ATP release from sweet receptor cells via cholinergic cell-to-cell (paracrine) signaling within a taste bud.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bachmanov AA et al (2001) Positional cloning of the mouse saccharin preference (sac) locus. Chem Senses 26:925–933
Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS (2010) Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci 123:972–982
Chaudhari N, Roper SD (2010) The cell biology of taste. J Cell Biol 190:285–296
Dando R, Roper SD (2012) Acetylcholine is released from taste cells, enhancing taste signaling. J Physiol 590:3009–3017
Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2012) Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci 32:322–330
Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N (2009) Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol 517:1–14
Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD (2011) GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci 31:5782–5791
Eguchi K, Ohtubo Y, Yoshii K (2008) Functional expression of M3, a muscarinic acetylcholine receptor subtype, in taste bud cells of mouse fungiform papillae. Chem Senses 33:47–55
Finger TE et al (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310:1495–1499
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Herness S et al (2002) Adrenergic signalling between rat taste receptor cells. J Physiol 543:601–614
Huang YJ et al (2007) The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104:6436–6441
Huang YA, Maruyama Y, Roper SD (2008) Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci 28:13088–13093
Huang YA, Dando R, Roper SD (2009) Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 29:13909–13918
Huang YA, Pereira E, Roper SD (2011) Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One 6:e25471
Huang YA, Grant J, Roper S (2012) Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS One 7:e30662
Kataoka S et al (2012) A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS One 7:e30032
Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A (2001) Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283:236–242
Krasteva G et al (2011) Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A 108:9478–9483
Kummer W, Lips KS, Pfeil U (2008) The epithelial cholinergic system of the airways. Histochem Cell Biol 130:219–234
Maruyama Y, Yasuda R, Kuroda M, Eto Y (2012) Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS One 7:e34489
Max M et al (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus sac. Nat Genet 28:58–63
McCaughey SA, Forestell CA, Tordoff MG (2005) Calcium deprivation increases the palatability of calcium solutions in rats. Physiol Behav 84:335–342
Montmayeur JP, Liberles SD, Matsunami H, Buck LB (2001) A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4:492–498
Nelson G et al (2001) Mammalian sweet taste receptors. Cell 106:381–390
Ninomiya Y, Tonosaki K, Funakoshi M (1982) Gustatory neural response in the mouse. Brain Res 244:370–373
Nofre G, Tinti JM, Chatzopoulos FO (1987) Sweetening agents. US4921939
Ogura T (2002) Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol 87:2643–2649
Ogura T, Lin W (2005) Acetylcholine and acetylcholine receptors in taste receptor cells. Chem Senses 30(Suppl 1):i41
Ogura T et al (2007) Immuno-localization of vesicular acetylcholine transporter in mouse taste cells and adjacent nerve fibers: indication of acetylcholine release. Cell Tissue Res 330:17–28
Ohsu T et al (2010) Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem 285:1016–1022
Romanov RA et al (2007) Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 26:657–667
Rybczynska A et al (2006) Hypertensive effect of calcilytic NPS 2143 administration in rats. J Endocrinol 191:189–195
Sainz E, Korley JN, Battey JF, Sullivan SL (2001) Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77:896–903
San Gabriel A, Uneyama H, Maekawa T, Torii K (2009) The calcium-sensing receptor in taste tissue. Biochem Biophys Res Commun 378:414–418
Tordoff MG et al (2008) Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics 34:338–348
Vandenbeuch A et al (2010) Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci 11:77
Acknowledgments
The author is grateful to Eriko Miura for performing excellent immunohistochemistry, technical assistance, and critical reading of the manuscript. I thank Toshihiko Hatanaka for synthesis of the kokumi substances, Yusuke Amino for synthesis of the sweetener, SC45647, and Hisayuki Uneyama for valuable discussions. I also thank Ken Iwatsuki of Tokyo University of Agriculture for valuable discussions. I thank Yi-jen A. Huang, Robin Dando, Nirupa Chaudhari, and Stephen D Roper of the University of Miami for valuable discussions and assistance.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Maruyama, Y. (2024). Molecular Mechanism of Enhancement in Basic Tastes by Kokumi Substances: A Potent Calcium-Sensing Receptor (CaSR) Agonist, γ-Glutamyl-Valinyl-Glycine, Amplifies Sweet-Induced ATP Secretion Via Cell-to-Cell Communication in a Mouse Taste Bud. In: Kuroda, M. (eds) Kokumi Substance as an Enhancer of Koku. Springer, Singapore. https://doi.org/10.1007/978-981-99-8303-2_9
Download citation
DOI: https://doi.org/10.1007/978-981-99-8303-2_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-8302-5
Online ISBN: 978-981-99-8303-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)