Abstract
At present, there are much radioactive waste oil temporarily stored in nuclear fuel processing plants, nuclear industry research institutes and operating nuclear power plants in China, which brings great storage pressure and safety risks to the operating nuclear facilities. In this paper, the components analysis of 40# waste oil used in nuclear facilities was carried out, and the elemental composition and chemical composition of the waste oil were obtained. The analysis showed that the main elements in the waste oil were C and H, and the main chemical components were alkanes, alkenes, aromatic hydrocarbons and alcohols with carbon chain length of 10–40. Using Aspen Plus software, the process flow model of waste oil’s steam reforming treatment was established. Based on the components analysis results of the waste oil, organic mixtures such as ethanol, ethane and propane were selected as the model components, and the element composition close to waste oil was obtained by adjusting the proportion of each component. The mixture was used as the source input of Aspen Plus to achieve good simulation results. The experimental results obtained under Pt catalyst at 400 ℃ were in good agreement with the simulation results, which confirmed the validity of the model. The thermodynamic equilibrium analysis of waste oil steam reforming reaction was carried out by using the verified model. The influence of reaction temperature (350–1150 ℃), pressure (0.01–100bar) and water to carbon ratio (0.01–100) on reforming reaction and off gas components in balanced state was studied. The conclusions are as follows: (1) The steam reforming reaction of waste oil has no obvious inhibition when the reaction pressure is less than 1bar, so the reforming reaction should be carried out under the condition of negative pressure less than 1bar; (2) The temperature should be maintained above 750 ℃ to ensure the complete steam reforming reaction; (3) Carbon deposition can be completely eliminated when the water/carbon ratio is higher than 1, and when the water/carbon ratio is higher than 10, the product components do not change with the water/carbon ratio.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keyword
1 Introduction
In the process of operation, maintenance and decommissioning of nuclear facilities, radioactive nuclides such as 137Cs, 90Sr and 60Co will be mixed into industrial oil to form radioactive waste oil [1]. The steam reforming treatment process developed by Studsvik is suitable for a variety of radioactive organic wastes, including waste oil, and has many advantages including high volume reduction ratio, stable solid products, and less environmental pollution [2]. Previously, Lin Li et al. from Nuclear Power Institute of China carried out a simulation on the steam reforming of waste resin and analyzed the balance products. They obtained the reaction parameters and operating gas velocity suitable for the treatment of radioactive waste resin [3,4,5]. However, there are few researches on steam reforming treatment of radioactive waste oil.
In the steam reforming process of waste oil, more hydrogen production is preferred, which is conducive to the subsequent off gas oxidation. Pressure, temperature and water-carbon ratio are the key parameters affecting the balanced off gas composition. A lot of experiments are needed to determine the appropriate reaction pressure, temperature and water-to-carbon ratio. But we can save a lot of effort by simulating the reaction process on the computer. Some researchers have carried out thermodynamic analysis on steam reforming of methanol [6, 7], ethanol [8,9,10], glycerol [11, 12] and other organic compounds, and obtained reforming reaction temperature, pressure and other operating parameters.
In this paper, Aspen Plus, a chemical process simulation software, was used to build a process model for oil steam reforming. In order to select the most suitable operating parameters for the oil steam reforming test, and to provide reference for the future engineering application of steam reforming process in the treatment of other radioactive wastes, the thermodynamic analysis of the oil steam reforming reaction was carried out and the effects of reaction temperature, pressure and water-carbon ratio on reforming reaction and off gas components was studied.
2 Oil Characterization
The feed composition is needed for thermodynamic analysis using Aspen Plus. 40# lubricating oil is widely used in various domestic nuclear facilities, thus we carried out the analysis of 40# oil from the aspects of elemental composition and chemical composition to provide input for Aspen Plus.
2.1 Elemental Composition
X-ray Fluorescence Spectrometer, Inductively Coupled Plasma Emission Spectrometer and electronic balance were used elemental composition analysis of 40# oil, and the mass fractions of carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur in the oil were measured. The results were shown in Table 1.
According to the analysis results, the elemental composition of the oil is approximately C35H66.8, omitting the very low content of N, O, P and S.
2.2 Chemical Composition
In this study, Agilent 7890–5977 GC/MSD was used to analyze the chemical composition of the oil. The chromatographic peak signal was compared with the NIST17 spectral database by computer to obtain the chemical composition. The results indicate that the oil is a mixture of various organic compounds, and its main chemical components are alkanes, alkenes, aromatic hydrocarbons and alcohols containing 10–40 carbon atoms. The main elements in waste oil are C and H, indicating that alkanes, alkenes, aromatic hydrocarbons and other hydrocarbons account for a relatively high proportion.
According to the above analysis results, the mixture of ethanol, ethane, propane, ethylene, benzene and toluene was selected as the feedstock, and the ratio (mole ratio) of each component of the mixture was 1:20:25:10:10:10. The mixture has an elemental and chemical composition similar to waste oil, and is suitable for the analysis of waste oil steam reforming reaction.
3 Simulation Modeling
The Aspen Plus model for waste oil steam reforming, as shown in Fig. 1, consists of three main operating units, PYRCT, CARBFLIT, ONSHT and RFRCT, along with some mixers and separators.
ONSHT is used to simulate steam generator and superheater. It preheats water vapor to reaction temperature and feeds the vapor into the reforming reactor RFRCT. RFRCT adopts the RGibbs reactor as the reactor model, which can calculate the product composition when the system reaches chemical equilibrium and phase equilibrium. PYRCT reactor is used to simulate the pyrolysis process of waste oil. The waste oil reaches pyrolysis equilibrium in the reactor to generate small-molecular-weight organic matter, hydrogen and carbon.
4 Results and Discussion
4.1 Effect of Reaction Pressure
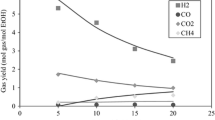
Figure 2 presents the variations of product concentrations versus reaction pressure. The reaction was carried out in 750 ℃ and steam/carbon ratio of 1.
At T = 750 ℃ and S/C = 1, methane steam reforming reaction and water gas conversion reaction are the main factors that affect the product composition. The concentration of H2 decreases with the increase of pressure. The composition of the product remains constant when the pressure is lower than 1 bar. However, methane steam reforming reaction is a reaction in which the number of gas molecules increases, and according to Le Chatelier's principle, when a system at equilibrium is subjected to a change in temperature, volume, concentration, or pressure, the system readjusts to partially counter the effect of the change, resulting in a new equilibrium. Therefore, as the pressure increases, methane steam reforming balance moves in the opposite direction, consuming CO and H2 to form CH4. When the pressure is higher than 1bar, the composition of the product is significantly affected by the pressure change. The concentration of CH4 in the product increases with the increase of water pressure, while the concentration of H2 and CO decreased respectively.
4.2 Effect of Reaction Temperature
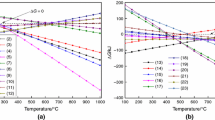
The influence of temperature can be seen from Fig. 3. Simulation is carried out at pressure of 1 bar and steam/carbon ratio of 1. Under these conditions, the mole fraction of hydrogen has significantly increased with increasing temperature up to 750 ℃. The increasing temperature shifts the reaction equilibrium toward the products side and thus producing more hydrogen.
The concentration of CO2 in reforming products tends to increase first and then decrease with the increase of temperature. When the temperature rises to 550 ℃, the concentration of CO2 reaches the peak. This is because the production of CO2 is mainly affected by the water-gas shift reaction, which is endothermic. Therefore, the rise of temperature has an inhibitory effect on the production of CO2. At low temperature, the reaction is weakly inhibited, and the mole fraction of CO also increases because the rise of temperature promotes the methane steam reforming reaction, which then shifts the equilibrium of the water-gas shift reaction to the direction of CO2 production. While above 550 ℃, the inhibition of high temperature on the water-gas conversion reaction is more obvious, so the content of CO2 gradually decreases with the increase of temperature.
4.3 Effect of Steam/carbon Ratio
The influence of steam/carbon ratio can be seen from Fig. 4. The operation temperature and pressure are fixed as 750 ℃, and 1 bar, respectively. The effect of steam/carbon ratio was investigated in the range of 0.01–100.
There is a large amount of carbon and no methane in the product at low steam/carbon ratio, which is the result of methane decomposition reactions. The water-gas shift reaction mainly occurs when the water-carbon ratio is between 0.01 and 1. Therefore, with the increase of the water-carbon ratio, the carbon decreases continuously until the ratio reaches 1, during which the H2 content also keeps rising. When the water-carbon ratio is higher than 0.1, the water-gas conversion reaction is encouraged, and the carbon dioxide content keeps rising, while the CO content reaches the peak when the water-carbon ratio is 1. Subsequently, as carbon is completely consumed, the increasing water vapor content will consume CO and generate CO2, and concentration remains constant when the water-carbon ratio is above 10.
5 Conclusions
In this paper, the waste oil was analyzed from the aspects of elemental composition and chemical composition. The analysis shows that the main elements of radioactive waste oil is C and H, and the contents of O, N, S and P are very low. Then Aspen Plus software was used to simulate the waste oil steam reforming reaction, and the thermodynamic equilibrium analysis of the reaction was carried out. The changes of product distribution in the reforming reaction were investigated when the reaction pressure was in the range of 0.01–100 bar, the temperature in 350–1150 ℃, and the water carbon ratio in 0.01–100. The following conclusions are drawn:
-
(1)
When the reaction pressure is less than 1bar, there is no obvious inhibition on the steam reforming reaction of waste oil, so the reforming reaction should be carried out under a negative pressure less than 1bar;
-
(2)
The temperature should be maintained above 750 ℃ to ensure the complete steam reforming reaction;
-
(3)
Carbon can be completely eliminated when the water/carbon ratio is higher than 1, and when the water/carbon ratio is higher than 10, the product components do not change with the water/carbon ratio.
References
Zhang, L., Xiong, Y.: Electrochemical advanced oxidation treatment of simulated radioactive waste oil . Southwest University of Science and Technology (2019)
Neeway, J.J., Jantzen, C.M., Brown, C.F., et al.: Radionuclide and contaminant immobilization in the fluidized bed steam reforming waste product. INTECH Open Access Publisher (2012)
Lin, L., Zhang, H., Li, W., et al.: Numerical simulation analysis on radioactive spent resin steam reforming fluidization based on VOF model. Sci. Technol. Eng. 20(30), 12657–12663 (2020)
Lin, L., Zhang, H., Li, W., et al.: Equilibrium product analysis of steam reforming waste resin based on gibbs free energy minimum principle. Sichuan Environ. 39(05), 170–174 (2020)
Lin, L., Chen, X., Li, W., et al.: Coupled numerical simulation of flow field reaction in a vertical tube for steam reforming of radioactive waste. Sci. Technol. Eng. 16(04), 200–204 (2016)
Amphlett, J., Evans, M., Jones, R., et al.: Hydrogen production by the catalytic steam reforming of methanol part 1: the thermodynamics. The Canadian J. Chem. Eng. 59(6), 720–727 (1981)
Faungnawakij, K., Kikuchi, R., Eguchi, K.: Thermodynamic evaluation of methanol steam reforming for hydrogen production. J. Power Sources 161(1), 87–94 (2006)
Garcia, E., Laborde, M.A.: Hydrogen production by the steam reforming of ethanol: thermodynamic analysis. Int. J. Hydrogen Energy 16(5), 307–312 (1991)
Rossi, C., Alonso, C., Antunes, O., et al.: Thermodynamic analysis of steam reforming of ethanol and glycerine for hydrogen production. Int. Jo.f hydrogen energy, 34(1), 323–332 (2009)
Fishtik, I., Alexander, A., Datta, R., et al.: A thermodynamic analysis of hydrogen production by steam reforming of ethanol via response reactions. Int. J. Hydrogen Energy 25(1), 31–45 (2000)
Chen, H., Zhang, T., Dou, B., et al.: Thermodynamic analyses of adsorption-enhanced steam reforming of glycerol for hydrogen production. Int. J. Hydrogen Energy 34(17), 7208–7222 (2009)
Adhikari, S., Fernando, S., Gwaltney, S.R., et al.: A thermodynamic analysis of hydrogen production by steam reforming of glycerol. Int. J. Hydrogen Energy 32(14), 2875–2880 (2007)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Wu, X. et al. (2023). Thermodynamic Equilibrium Analysis of Steam Reforming Reaction of Radioactive Waste Oil. In: Liu, C. (eds) Proceedings of the 23rd Pacific Basin Nuclear Conference, Volume 1. PBNC 2022. Springer Proceedings in Physics, vol 283. Springer, Singapore. https://doi.org/10.1007/978-981-99-1023-6_97
Download citation
DOI: https://doi.org/10.1007/978-981-99-1023-6_97
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-1022-9
Online ISBN: 978-981-99-1023-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)