Abstract
Termites are the major ecosystem service providers and contribute significantly to soil processes and nutrient cycling in tropical ecosystems. The ecological services provided by termites are often discredited due to their commonly-regarded status as pest in human-dominated landscapes, however. In order to understand the potential roles of termites in peatland ecosystems, termite samplings were conducted in abandoned degraded peatland and peatland cultivated with oil palm in Riau, Sumatra. Surveys found a total of six species of termite of the family Rhinotermitidae. (rhinotermitid) in study plots of disturbed lands. In particular, Coptotermes spp. are notorious pests to oil palm, and may also be a potential pest in indigenous tree replanting programs. Based on analysis of termite feeding groups and documentation of wood susceptibility to termite attack, this study provides a reference of tree species that must be avoided in indigenous tree replanting programs so that the trophic relations of termite populations are of most benefit to peatland soil biodiversity and thereby to resilient peatland ecosystems.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Insects contribute significantly to vital ecological functions such as pollination, decomposition, maintenance of wildlife species, and as biological control agent for crop pests (Losey and Vaughan 2006). At the same time, insects can be pests. If they appear at the wrong place or time they can threaten economic wellbeing and human health. This is particularly true when their natural habitat is disturbed by urbanization or other landscape modification, and when they are mismanaged.
Termites are major ecosystem service providers and contribute significantly in soil processes and nutrient cycling in tropical ecosystem (Holt and Lepage 2000). This vital role in ecological processes is often underacknowledged, as 5% of the 2300 termite species in the world are pests known to have negative economic impact on human settlements or agriculture (Su and Scheffrahn 2000). Recent increases in land-use intensity have elevated termites’ pest status.
The Indo-Malayan region contains 62% of global tropical peatlands (Page et al. 2006). These peatlands have long served as a biodiversity hotspot for many endemic species of flora, fauna, and microbes (Yule 2010). Peatland ecosystems have also supported local community livelihoods based on long-term coexistence with peatland forests. Political policy, urbanization, and agricultural intensification, which came on the heels of increased global demand for food and fuel, resulted in the clearing of 80% of Southeast Asian peat swamp forest to make way for agro-industrial plantations (Mishra et al. 2021). In the early 2000s, 6% of tropical peatlands in the Indo-Malayan region was converted to oil-palm plantations (Koh et al. 2011), as abandoned secondary peatland increased 15% since the 1990s (Miettinen and Liew 2010). Both processes of land modification pose considerable risks to biodiversity. Even such little-regarded species as earthworms, which make similar contributions to soil processes as do termites, are disturbance-sensitive (Blanchart and Julka 1997). Owing to the unusual peat ecosystem that is highly acidic, anaerobic, and sensitive to fire and disturbed conditions, earthworms are nearly absent from disturbed peat (Cotton and Curry 1982). Termites, which tend to be much more resilient to land disturbance than are earthworms and other related species, could therefore be seen as a major soil engineer if their services could be harnessed efficiently.
As a first step toward understanding the potential roles of termites in the peat system, this study conducted termite samplings in abandoned degraded peatland and peatland cultivated with oil palm in Riau, Sumatra. The authors also combined the dataset of previous reports in disturbed and peatlands cultivated with oil palm. Based on analysis of termite feeding groups and documentation of wood susceptibility to termite attack, this study provides a reference of tree species that must be avoided in indigenous tree replanting programs so that the trophic relations of termite populations are of most benefit to peatland soil biodiversity and thereby to resilient peatland ecosystems.
2 Materials and Methods
An abandoned degraded peatland site and a peatland currently cultivated with 5–6 year old oil palm were selected for this study (Fig. 6.1). The sites are located in the transition zone of the GiamSiak Kecil–Bukit Batu Biosphere Reserve (0°44′–1°11′N and 0°11′–102°10′E) lying between 0 and 50 m above sea level. The study sites were 2 km apart, allowing the authors to exclude any major discrepancy in hydrological and climatic patterns, with the principal exception of canopy openness. The canopy cover in oil palm plantation registered an average of 37.8% ± 14.1 (±SE); in contrast, the abandoned degraded peatland was open shrubland.
Termite samplings were carried out in November 2012 using a standardized belt transect as described by Jones and Eggleton (2000). The belt transect comprised a survey area of 100 × 2 m and was divided into 20 sections of 5 × 2 m. A collector spent an hour on each section to collect the soldiers and worker termites from termite potential habitats such as dead tree branches, tree logs, soil under logs, termite galleries and nests. Collected termites were stored in 80% ethanol until identified. The termites were sorted to species level based on identification keys on the termite fauna in the Indo-Malayan region (Thapa 1977; Tho 1992; Gathorne-Hardy 2004).
Termite species richness was estimated using Chao 2, Incidence-based coverage estimator (ICE) and Jackknife 1, and the diversity indices (Shannon and Simpson Indices) were generated using EstimateS Version 8.2 (Colwell 2009). The relative abundance of termites was generated based on the encounter rates of termite over 20 sections. The relative abundance in abandoned land is relative to the value in oil palm cultivated land transect (relative abundance = 1).
3 Results
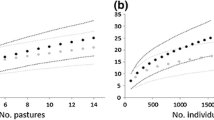
The observed species richness accumulation curve for both study sites reached an obvious asymptote close to estimated species richness curves (i.e., Chao 2, Incidence-based coverage estimator and Jackknife 1) (Fig. 6.2). In addition, all termite species were consistently encountered throughout the sampling as evidenced from no termite species only found once or twice (Singletons = 0; Doubletons = 0).
Species accumulation curves of termites sampled in abandoned degraded peatland and peatland cultivated with oil palm in Riau, Indonesia. Species observed is the number of termites that were collected in the 100 m belt transect of 20 sections, and the numbers of termite species that were beyond observation were predicted using Chao 2, ICE and Jackknife 1
A total of six species of termite, which comprised a single family Rhinotermitidae, two subfamilies (i.e., Rhinotermitinae and Coptotermitinae) and three genera (i.e., Coptotermes, Schedorhinotermes, and Parrhinotermes) were collected in both study sites. Coptotermes gestroi (Wasmann) was found only in abandoned degraded peatland, but Parrhinotermes pygmaues (John) and Schedorhinotermes malaccensis (Holmgren) were absent compared to that termite assemblage in degraded peat land cultivated with oil palm (Table 6.1).
Overall, the relative abundance of termites in abandoned degraded peatland was 65% of that in degraded peatland cultivated with oil palm (Fig. 6.3). In the abandoned degraded peat Coptotermes kalshoveni (Kemner), Parrhinotermes aequalis (Havilandi) and Schedorhinotermes medioobscurus (Holmgren) were predominately found (Shannonexp index: 3.34; Simpson index: 3.45), with relative abundances of 23%, 41%, and 32%, respectively. In contrast, the termite dominance in oil palm is even (Shannonexp index: 4.93; Simpson index: 5.50). Each species registered approximately 20% of encounter rate in the transect sampling (Table 6.2). All termite species found were wood feeders and wood-nesting termites.
4 Discussion
The termite assemblage in the transition zones of the GiamSiak Kecil-Bukit Batu Biosphere Reserve contained only a single family of lower termite–Rhinotermitidae (rhinotermitids), which consist of two subfamilies (Coptotermitinae and Rhinotermitinae), three genera, and six termite species. The result accords closely with the termite assemblage sampled in newly cleared land (Vaessen et al. 2011). The low termite-species richness in the present study sites might be the result of land disturbance during the clearing process or repeated fires, causing the collapse of susceptible termite species. Only wood-nesting termites survived at post disturbance (Neoh et al. 2016). However, this result needs to be viewed with caution, as it was based only on a single landscape. A study at a 2 year-old oil plantation conversion from mixed swamp forest in Sri Aman District, Sarawak, documented two subfamilies of higher termites (i.e., Nasutitermitinae and Termitinae) and a subfamily of lower termite (Rhinotermitinae) (Vaessen et al. 2011). Another study of 5–7 year-old and 13–15 year-old oil palm plantation sites recorded more than 12 species, which comprised two subfamilies of lower termites (Coptotermitinae and Rhinotermitinae), and four subfamilies of higher termites (Nasutermitinae, Termitinae, Amitermitinae, and Macrotermitinae [only found in 15–17 year-old plot]). These findings indicate that the assemblage of termites shifts from a single dominant family to multiple families (i.e., rotten-woody feeder and soil feeder) along with the age of plantation. Kon et al. (2012) attributed this phenomenon to the improvement of soil condition across time, which favored the termite colony and territory expansion. In addition, incremental increases of dead wood, woody-plant basal area, and canopy height (as the plantation aged) may also be associated with the presence of multiple woody and soil feeders taxa in disturbed areas (Jones et al. 2003).
Termite species richness and relative abundance typically decline following logging and land-clearing activity (Jones et al. 2003). The lack of termite assemblage in burned peatland may have reduced the functional groups of termites that are essential to soil processes. Okwakol (2000), who studied land conversion from natural forest to banana-cultivated land, has suggested that a drastic reduction of 40% of the original termite diversity potentially alters ecological processes and reduces farmland productivity over time. In the present study, termite species richness fell to one family, Rhinotermitidae. It is still difficult to estimate what rates of termite abundance and diversity can elicit significant ecosystem function in a given area. However, termite groups with proven ecosystem functionality (e.g., Macrotermitinae [Beaudrot et al. 2011], Nasutitermitinae [Jiménez et al. 2008]) were not found in our study sites. Several management practices are therefore required to maintain termite diversity when peatland is disturbed. These management techniques include: (a) using low-impact land-clearing techniques and leaving tree residues in the disturbed area (rather than burning them) in order to maintain termite populations (Bourguignon et al. 2018); and (b) maintaining patches of intact forest within a disturbed area (Neoh et al. 2017). These methods would promote the survival of termite species vulnerable to land disturbance and help restore termite biodiversity in degraded lands (Jones et al. 2003).
In mineral soils, termites are known to make subterranean tunnels that improve soil water infiltration rates, while their role in decomposing plant litter and mound building via soil translocation enriches soil nutrients and improves soil bulk density, respectively (Holt and Lepage 2000). In the Indo-Malayan region, the termite assemblage in peatlands is mainly comprised of decayed-wood feeders and soil feeders (Table 6.1). Black and Okwakol (1997) have noted that wood and soil feeders generally contain high levels of organic carbon in their nests; the latter’s nest, in particular, is rich in nitrogen due to their habit of feeding on highly decaying soil-like cellulose materials. A study on soil-feeding termites in the Colombian savanna revealed that the group was reputedly more important in soil nutrient enrichment than ants (Jiménez et al. 2008). Studies on soil impact by Nasutitermes sp. revealed that termite mounds can act as source of nitrogen available to plants in nutrient-depleted savanna systems (López-Hernández 2001; Jiménez et al. 2006). The presence of other feeding groups in the peat system (Table 6.1) may also be vital in the optimal functioning of the ecosystem and peat restoration.
In savanna systems, grass-harvesting termites are believed to play an important role in fire management, especially when they remove grass and plant litter that could act as fuel during fire events (Davies et al. 2010). Though grass-harvesting termites are not found in the Indo-Malayan region, the presence of wood feeders of multiple taxa potentially reduces the availability of fuel materials in the peatlands.
Fire disturbance and land conversion in the peatlands under study have caused a drastic collapse in the termite assemblage, leaving only rhinotermitids behind. Although the termite assemblages reported by Vaessen et al. (2011) and Kon et al. (2012) were diverse, the relative abundance of rhinotermitids in a young oil palm plantation of 5–7 years-old was generally high compared those found of other families, as rhinotermitids account for more than 70% of the total termite encounter.
Coptotermes are highly destructive termite species in Southeast Asia. Studies conducted on oil palm growth in peatlands in peninsular Malaysia and Sarawak reported that Coptotermes curvinagthus (Holmgren) is a major pest that causes death in both mature and young oil palm trees (Kim Huan and Silek 2001; Cheng et al. 2008; Kon et al. 2012). Similarly, approximately 14% of rubber tree planted on peat soil were attacked by C. curvignathus (Indrayani et al. 2022). Rasmussen et al. (1982) suggested that an abundance of food sources from the incomplete removal of timber residue during forest clearing could account for the high prevalence of C. curvinagthus attack on new plantation plots. This premise has been discounted, however, as no clear relationship between the termite attack and plant residue was found in an oil palm plantation on mineral soil (Kirton et al. 1999), which could be the result of the dominance and resilience of C. curvignathus in newly cleared land (Cheng et al. 2008). Coptotermes sepangensis Krishna, C. borneensis Oshima, C. gestroi, and C. kalshoveni were also found in oil palm plantations, but the species generally nest in rotten wood and rarely cause tree death. Nevertheless, C. gestroi and C. kalshoveni are notorious for damaging building structures in the tropics (Kirton and Azmi 2005). Other termite species Schedorhinotermes spp., Globitermes globosus (Havilandi), and G. sulphurues (Havilandi) were occasional pests in oil palm plantations (Harris 1969). Their pest status may be elevated if their food sources and habitats are further disturbed.
Replanting fast-growing indigenous tree species in abandoned degraded peatland such as Dyera lowii, Tetramerista glabra, Palaquium sumatranum, Palaquium burckii, Cratoxylon arborescens, and Callophyllum lowii is favored in peat rehabilitation programs; it can also be a key element in local community activities and source of livelihood (Gunawan et al. 2011, 2013). Nevertheless, some of these tree species are susceptible to termite attack (Table 6.3). Thus we do not rule out potential termite attacks in community-planted forests, just as have occurred in oil palm plantations. To ensure the survival and growth of newly-planted trees, farmers must take into account species selection and wood resistance to termite attacks. Otherwise, sustainable pest management and preventive measures should be devised before termites become a serious threat to newly re-planted peatlands.
5 Conclusion
In Southeast Asia, termites are generally thought of as pests, and their ecological services to farmlands is underappreciated. With little awareness of the potentially vital role in nutrient management these soil-dwelling insects could play, farmers have often applied chemical pesticides to control termite populations in peatland oil palm plantations. Farmers drench the soil with chemicals or directly spray infestations. In most cases, these methods are repeatedly applied, as termite infestations generally reoccur after the chemical effect dissipates. Such intensive chemical interventions have wider impact on soil communities; they do not only kill pest termite species, i.e., C. curvignathus, but also beneficial wood- and soil-feeding termites as well as other arthropods (e.g., beetles and ants) providing natural ecosystem services. It is interesting to note that farmers in Africa possess comprehensive knowledge of termite ecology, termite species, and utilize various traditional eco-friendly control practices (Sileshi et al. 2009). Our study demonstrates that sustainable pest management and preventive measures such as tree-selection programs should be devised before termites pose a serious threat to newly planted trees.
Referecses
Arinana, Tsunoda K, Herliyana EN et al (2012) Termite-susceptible species of wood for inclusion as a reference in Indonesian standardized laboratory testing. Insects 3(2):396–401. https://doi.org/10.3390/insects3020396
Beaudrot L, Du Y, Rahman Kassim A et al (2011) Do epigeal termite mounds increase the diversity of plant habitats in a tropical rain forest in Peninsular Malaysia? PLoS One 6(5):19777. https://doi.org/10.1371/journal.pone.0019777
Black HIJ, Okwakol MJN (1997) Agricultural intensification, soil biodiversity and agroecosystem function in the tropics: the role of termites. Appl Soil Ecol 6(1):37–53. https://doi.org/10.1016/S0929-1393(96)00153-9
Blanchart E, Julka JM (1997) Influence of forest disturbance on earthworm (Oligochaeta) communities in the Western Ghats (South India). Soil Biol Biochem 29(3–4):303–306. https://doi.org/10.1016/S0038-0717(96)00094-6
Bourguignon T, Dahlsjö CAL, Salim KA et al (2018) Termite diversity and species composition in heath forests, mixed dipterocarp forests, and pristine and selectively logged tropical peat swamp forests in Brunei. Insect Soc 65(3):439–444. https://doi.org/10.1007/s00040-018-0630-y
Cheng S, Kirton LG, Gurmit S (2008) Termite attack on oil palm grown on peat soil: identification of pest species and factors contributing to the problem. Planter 84(991):200–210
Colwell RK (2009) EstimateS 8.2.0.: statistical estimation of species richness and shared species from samples. User's guide and application. Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs
Cotton DCF, Curry JP (1982) Earthworm distribution and abundance along a mineral-peat soil transect. Soil Biol Biochem 14(3):211–214. https://doi.org/10.1016/0038-0717(82)90026-8
Davies AB, Parr CL, Van Rensburg BJ (2010) Termites and fire: current understanding and future research directions for improved savanna conservation. Austral Ecol 35(4):482–486. https://doi.org/10.1111/j.1442-9993.2010.02124.x
Donovan SE, Eggleton P, Bignell DE (2001) Gut content analysis and a new feeding group classification of termites. Ecol Entomol 26(4):356–366. https://doi.org/10.1046/j.1365-2311.2001.00342.x
Dungani R, Islam MN, Abdul Khalil HPS et al (2013) Termite resistance study of oil palm trunk lumber (OPTL) impregnated with oil palm shell meal and phenol-formaldehyde resin. Bioresources 8(4):4937–4950
Faszly R, Mohammad-Faris ME, Azizil-Alimin MM et al (2011) Termites of oil palm on peat soil: a 10-year collection from Endau Rompin. Serangga 16(2):37–56
Gathorne-Hardy FJ (2004) The termites of Sundaland: a taxonomic review. Sarawak Museum J 60(81):89–133
Gunawan H, Kobayashi S, Mizuno K et al (2011) Progress on restoration experiments of degraded peat swamp forest ecosystem in the Giam Siak Kecil-Bukit Batu Biosphere Reserve, Riau, Indonesia. In: Purwanto Y, Mizuno K (eds) Proceeding of the international workshop on sustainable management of bio-resources in tropical peat-swamp forest, Cibinong, 2011
Gunawan H, Kobayashi S, Mizuno K et al (2013) Sustainable rehabilitation of tropical peat swamp forest ecosystem in Giam Siak Biosphere Reserve, Riau, Indonesia: an integrated approach. In: Ishimaru K, Kobayashi S (eds) Proceedings of international workshop on incentive of local community for REDD and semi-domestication of non-timber forest products, Kyoto, 2011
Hadi YS (2014) Feeding rate as a consideration factor for successful termite wood preference tests. In: Forschler BT (ed) Proceedings of the 10th pacific termite research group conference, Kuala Lumpur, 2014
Hadi YS, Massijaya MY, Hadjib N et al (2012) The resistance of six Papua New Guinea woods to subterranean termite attack. In: Proceedings of the 9th Pacific-Rim termite research group conference, Honoi, February 2012. Science and Technics Publishing House, Hanoi, pp 91–94
Hadjib N, Massijaya MY, Hadi YS et al (2012) Resistance of three small diameter logs to subterranean termite attack. In: Proceedings of the 9th Pacific-Rim termite research group conference, Honoi, February 2012. Science and Technics Publishing House, Hanoi, pp 95–98
Harris WV (1969) Termites as pests of crops and trees. Commonwealth Agricultural Bureau, London
Holt JA, Lepage M (2000) Termites and soil properties. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, pp 389–407. https://doi.org/10.1007/978-94-017-3223-9_18
Indrayani Y, Setyawati D, Maryani Y et al (2022) A termite attack on rubber plantation on peat soil: level of damage and identification of pest species. In: 2nd international conference on tropical wetland biodiversity and conservation, Banjarbaru City, October 2021. IOP conference series: earth and environmental science, vol 976. IOP Publishing, Bristol, 012006. https://doi.org/10.1088/1755-1315/976/1/012006
Jalaludin NA, Rahim F, Yaakop S (2018) Termite associated to oil palm stands in three types of soils in Ladang Endau Rompin, Pahang, Malaysia. Sains Malays 47(9):1961–1967. https://doi.org/10.17576/jsm-2018-4709-03
Jasni HR, Rachman O (2009) The resistance of pine wood from timber estate against termite at various levels of tree age. In: Proceedings of the 6th conference of the Pacific Rim termite research group, Kyoto, 2–3 March 2009
Jiménez JJ, Decaëns T, Lavelle P (2006) Nutrient spatial variability in biogenic structures of Nasutitermes (Termitinae; Isoptera) in a gallery forest of the Colombian ‘Llanos’. Soil Biol Biochem 38(5):1132–1138. https://doi.org/10.1016/j.soilbio.2005.09.026
Jiménez JJ, Decaëns T, Lavelle P (2008) C and N concentrations in biogenic structures of a soil-feeding termite and a fungus-growing ant in the Colombian savannas. Appl Soil Ecol 40(1):120–128. https://doi.org/10.1016/j.apsoil.2008.03.009
Jones DT, Eggleton P (2000) Sampling termite assemblages in tropical forests: testing a rapid biodiversity assessment protocol. J Appl Ecol 37(1):191–203. https://doi.org/10.1046/j.1365-2664.2000.00464.x
Jones DT, Susilo FX, Bignell DE et al (2003) Termite assemblage collapse along a land-use intensification gradient in lowland Central Sumatra, Indonesia. J Appl Ecol 40(2):380–391. https://doi.org/10.1046/j.1365-2664.2003.00794.x
Kim Huan L, Silek B (2001) Termite infestation on oil palms planted on deep peat in Sarawak: tradewinds experience. In: Cutting-edge technologies for sustained competitiveness. Proceedings of the 2001 PIPOC international palm oil congress, Kuala Lumpur, August 2001. Malaysian Palm Oil Board, Selangor, pp 355–368
Kirton LG, Azmi M (2005) Patterns in the relative incidence of subterranean termite species infesting buildings in Peninsular Malaysia. Sociobiology 46(1):1–15
Kirton LG, Brown VK, Azmi M (1999) Do forest-floor wood residues in plantations increase the incidence of termite attack?—testing current theory. J Trop For Sci 11:218–239
Koh LP, Miettinen J, Liew SC et al (2011) Remotely sensed evidence of tropical peatland conversion to oil palm. PNAS 108(12):5127–5132. https://doi.org/10.1073/pnas.1018776108
Kon TW, Bong CF, King JH et al (2012) Biodiversity of termite (Insecta: Isoptera) in tropical peat land cultivated with oil palms. Pak J Biol Sci 15(3):108–120. https://doi.org/10.3923/pjbs.2012.108.120
López-Hernández D (2001) Nutrient dynamics (C, N and P) in termite mounds of Nasutitermes ephratae from savannas of the Orinoco Llanos (Venezuela). Soil Biol Biochem 33(6):747–753. https://doi.org/10.1016/S0038-0717(00)00220-0
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56(4):311–323. https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Miettinen J, Liew SC (2010) Degradation and development of peatlands in Peninsular Malaysia and in the islands of Sumatra and Borneo since 1990. Land Degrad Dev 21(3):285–296. https://doi.org/10.1002/ldr.976
Mishra S, Page SE, Cobb AR et al (2021) Degradation of Southeast Asian tropical peatlands and integrated strategies for their better management and restoration. J Appl Ecol 58(7):1370–1387. https://doi.org/10.1111/1365-2664.13905
Neoh KB, Bong LJ, Muhammad A et al (2016) The impact of tropical peat fire on termite assemblage in Sumatra, Indonesia: reduced complexity of community structure and survival strategies. Environ Entomol 45(5):1170–1177. https://doi.org/10.1093/ee/nvw116
Neoh KB, Bong LJ, Muhammad A et al (2017) The effect of remnant forest on insect successional response in tropical fire-impacted peatland: a bi-taxa comparison. PLoS One 12(3):e0174388. https://doi.org/10.1371/journal.pone.0174388
Okwakol MJN (2000) Changes in termite (Isoptera) communities due to the clearance and cultivation of tropical forest in Uganda. Afr J Ecol 38(1):1–7. https://doi.org/10.1046/j.1365-2028.2000.00189.x
Page SE, Rieley JO, Wüst R (2006) Lowland tropical peatlands of Southeast Asia. In: Martini IP, Martínez Cortizas A, Chesworth W (eds) Peatlands: evolution and records of environmental and climate changes, Developments in earth surface processes, vol 9. Elsevier, Amsterdam, pp 145–172. https://doi.org/10.1016/S0928-2025(06)09007-9
Rasmussen AN, Kanapathy K, Santa Maria N et al (1982) Establishment of oil palm on deep peat from jungle. In: Pushparajah E, Chew PS (eds) The oil palm in agricultural in the eighties. The Incorporated Society of Planters, Kuala Lumpur, pp 641–651
Sileshi GW, Nyeko P, Nkunika POY et al (2009) Integrating ethno-ecological and scientific knowledge of termites for sustainable termite management and human welfare in Africa. Ecol Soc 14(1):48
SNI (Standar Nasional Indonesia) (2006) SNI 01.7207-2006: Uji ketahanan kayu dan produk kayu terhadap organisme perusak kayu. Badan Standardisasi Nasional, Jakarta
Su NY, Scheffrahn RH (2000) Termites as pest of buildings. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, pp 437–453. https://doi.org/10.1007/978-94-017-3223-9_20
Thapa RS (1977) Termites of Sabah. Sabah Forestry Department, Sandakan
Tho YP (1992) Termites of peninsular Malaysia. Forest Research Institute of Malaysia, Kuala Lumpur
Vaessen T, Verwer C, Demies M et al (2011) Comparison of termite assemblages along a landuse gradient on peat areas in Sarawak, Malaysia. J Trop For Sci 23(2):196–203
Wardani L, Subaru D, Jasni et al (2009) Termite resistance of some woods from natural and plantation forests in South Kalimantan Indonesia. In: Proceedings of the 6th Pacific Rim termite research group conference, Kyoto, 2–3 March 2009
Wardani L, Risnasari I, Yasni Hadi YS et al (2012) Resistance of Jabon timber modified with styrene and MMA against soil termites and dry wood termites. In: Proceedings of the 9th Pacific- Rim Termite Research Group Conference, Science and Technics Publishing House, Hanoi, pp 73–78
Yule CM (2010) Loss of biodiversity and ecosystem functioning in Indo-Malayan peat swamp forests. Biodivers Conserv 19(2):393–409. https://doi.org/10.1007/s10531-008-9510-5
Acknowledgments
We thank Mr. Nur who kindly provided accommodations during our field survey. K.-B.N. was an international researcher fellow of the Japan Society for the Promotion of Science. This study was partly funded by Large-Scale Research Program “Promoting the Study of Sustainable Humanosphere in Southeast Asia” funded by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT), 2011–2016, and the institutional collaboration feasibility studies “Toward the Regeneration of Tropical Peatland Societies: Building International Research Network on Paludiculture and Sustainable Peatland Management”, Research Institute for Humanity and Nature (RIHN).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Neoh, KB., Muhammad, A., Itoh, M., Kozan, O. (2023). Termite: Friend or Foe? Conservation Values of Termites in Tropical Peat Systems. In: Mizuno, K., Kozan, O., Gunawan, H. (eds) Vulnerability and Transformation of Indonesian Peatlands. Global Environmental Studies. Springer, Singapore. https://doi.org/10.1007/978-981-99-0906-3_6
Download citation
DOI: https://doi.org/10.1007/978-981-99-0906-3_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0905-6
Online ISBN: 978-981-99-0906-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)