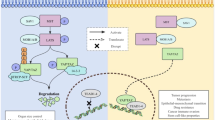
Abstract
Lung cancer produces the highest mortality rate among cancers, mainly due to its late diagnosis accompanied by single or multiple organ metastasis to the lymph node, pleura, liver, brain and/or bones. In this chapter, we will review the major cellular signalling pathways that promote the proliferation and metastasis of lung cancer cells to each of these metastatic sites, hopefully to shine the light on the possible therapeutic targets that could be developed against these pathways for better therapeutic outcome. Cellular pathways including MAPK, CXCR4/CXCL12, ALK, Rho/ROCK, PI3K and RANK/RANKL signalling will be elaborated in relation to their roles in lymph node, pleura, liver, brain and bone metastasis of lung cancer, respectively. The blooming understanding of microRNA (miRNA) in lung cancer in these recent years has shown that it could play bi-faceted roles in modulating oncogenic pathways. Thus, we also briefly described the candidates of miRNA that may either promote or inhibit the key pathways in lung cancer with various metastatic sites.
Both Siti Fathiah Masre and Amnani Aminuddin are first authors of the book chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Petersen I (2011) The morphological and molecular diagnosis of lung cancer. Dtsch Aerzteblatt Online 108:525–531. https://doi.org/10.3238/arztebl.2011.0525
Rutering J, Ilmer M, Recio A et al (2016) Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat Med 5:1–8. https://doi.org/10.4172/2157-7633.1000305.Improved
Popper HH (2016) Progression and metastasis of lung cancer. Cancer Metastasis Rev 35:75–91. https://doi.org/10.1007/s10555-016-9618-0
Shin DY, Na II, Kim CH et al (2014) EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 9:195–199. https://doi.org/10.1097/JTO.0000000000000069
Tamura T, Koichi K, Kensuke N et al (2015) Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol 3:217–221. https://doi.org/10.3892/mco.2014.410
Wilbertz T, Wagner P, Petersen K et al (2011) SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol 24:944–953. https://doi.org/10.1038/modpathol.2011.49
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio Cancer Genomics Portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404. https://doi.org/10.1158/2159-8290.CD-12-0095
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6(269):p11. https://doi.org/10.1126/scisignal.2004088
Wang S, Wang Z (2015) Meta-analysis of epidermal growth factor receptor and KRAS gene status between primary and corresponding metastatic tumours of non-small cell lung cancer. Clin Oncol 27:30–39. https://doi.org/10.1016/j.clon.2014.09.014
World Health Organization (2019) European tobacco use: trends report 2019
Hasegawa Y (2014) Lung cancer: progress in diagnosis and treatments. Topics: I. Epidemiology and pathogenesis; 2. The etiology of lung cancer. Nihon Naika Gakkai Zasshi 103:1261–1266. https://doi.org/10.2169/naika.103.1261
Thun MJ, Carter BD, Feskanich D et al (2013) 50-Year trends in smoking-related mortality in the United States. N Engl J Med 368:351–364. https://doi.org/10.1056/NEJMsa1211127
Nasim F, Sabath BF, Eapen GA (2019) Lung cancer. Med Clin North Am 103:463–473. https://doi.org/10.1016/j.mcna.2018.12.006
O’Malley M, King AN, Conte M et al (2014) Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol 9:917–926. https://doi.org/10.1097/JTO.0000000000000191
Bianchi F, Nicassio F, Marzi M et al (2011) A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol Med 3:495–503. https://doi.org/10.1002/emmm.201100154
Huang W, Hu J, Yang DW et al (2012) Two microRNA panels to discriminate three subtypes of lung carcinoma in bronchial brushing specimens. Am J Respir Crit Care Med 186:1160–1167. https://doi.org/10.1164/rccm.201203-0534OC
Lu Y, Govindan R, Wang L et al (2012) MicroRNA profiling and prediction of recurrence/relapse-free survival in stage I lung cancer. Carcinogenesis 33:1046–1054. https://doi.org/10.1093/carcin/bgs100
Wu KL, Tsai YM, Lien CT et al (2019b) The roles of microRNA in lung cancer. Int J Mol Sci 20(7):1611
Wu SG, Chang TH, Liu YN, Shih JY (2019c) MicroRNA in lung cancer metastasis. Cancers (Basel) 11:265
Dillekås H, Rogers MS, Straume O (2019) Are 90% of deaths from cancer caused by metastases? Cancer Med 8:5574–5576. https://doi.org/10.1002/cam4.2474
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Paduch R (2016) The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol 39:397–410. https://doi.org/10.1007/s13402-016-0281-9
Mani SA, Guo W, Liao MJ et al (2008) The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 133:704–715. https://doi.org/10.1016/j.cell.2008.03.027
Liu XG, Zhu WY, Huang YY et al (2012) High expression of serum miR-21 and tumor miR-200c associated with poor prognosis in patients with lung cancer. Med Oncol 29:618–626. https://doi.org/10.1007/s12032-011-9923-y
Wu C, Cao Y, He Z et al (2014) Serum levels of miR-19b and miR-146a as prognostic biomarkers for non-small cell lung cancer. Tohoku J Exp Med 232:85–95. https://doi.org/10.1620/tjem.232.85
Zhang J, Wang T, Zhang Y et al (2018) Upregulation of serum miR-494 predicts poor prognosis in non-small cell lung cancer patients. Cancer Biomark 21:763–768. https://doi.org/10.3233/CBM-170337
Osugi J, Kimura Y, Owada Y et al (2015) Prognostic impact of hypoxia-inducible miRNA-210 in patients with lung adenocarcinoma. J Oncol 2015:1–8. https://doi.org/10.1155/2015/316745
Li Y, Cui X, Li Y et al (2018a) Upregulated expression of miR-421 is associated with poor prognosis in non-small-cell lung cancer. Cancer Manag Res 10:2627–2633. https://doi.org/10.2147/CMAR.S167432
Wang SY, Li Y, Jiang YS, Li RZ (2017a) Investigation of serum miR-411 as a diagnosis and prognosis biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol Sci 21:4092–4097
Han L, Zhang G, Zhang N et al (2014) Prognostic potential of microRNA-138 and its target mRNA PDK1 in sera for patients with non-small cell lung cancer. Med Oncol 31:1–7. https://doi.org/10.1007/s12032-014-0129-y
Yang ZQ, Wu CA, Cheng YX (2018) Prognostic Value of microRNA-133a expression and its clinicopathologic significance in non-small cell lung cancer: a comprehensive study based on meta-analysis and the tcga database. Oncol Res Treat 41:762–768. https://doi.org/10.1159/000492343
Gan X, Luo J, Tang R et al (2017b) Clinical value of miR-452-5p expression in lung adenocarcinoma: a retrospective quantitative real-time polymerase chain reaction study and verification based on The Cancer Genome Atlas and Gene Expression Omnibus databases. Tumor Biol 39:101042831770575. https://doi.org/10.1177/1010428317705755
Gan TQ, Xie ZC, Tang RX et al (2017a) Clinical value of miR-145-5p in NSCLC and potential molecular mechanism exploration: a retrospective study based on GEO, qRT-PCR, and TCGA data. Tumor Biol 39:1–23. https://doi.org/10.1177/1010428317691683
Lu HM, Yi WW, Ma YS et al (2018) Prognostic implications of decreased microRNA-101-3p expression in patients with non-small cell lung cancer. Oncol Lett 16:7048–7056. https://doi.org/10.3892/ol.2018.9559
Li J, Tan Q, Yan M et al (2014) MiRNA-200c inhibits invasion and metastasis of human non-small cell lung cancer by directly targeting ubiquitin specific peptidase 25. Mol Cancer 13:166. https://doi.org/10.1186/1476-4598-13-166
Hou L, Luo P, Ma Y et al (2017) MicroRNA-125a-3p downregulation correlates with tumorigenesis and poor prognosis in patients with non-small cell lung cancer. Oncol Lett 14:4441–4448. https://doi.org/10.3892/ol.2017.6809
Yu X, Wei F, Yu J et al (2015) Matrix metalloproteinase 13: a potential intermediate between low expression of microRNA-125b and increasing metastatic potential of non-small cell lung cancer. Cancer Genet 208:76–84. https://doi.org/10.1016/j.cancergen.2015.01.006
Wang XC, Tian LL, Wu HL et al (2010) Expression of miRNA-130a in nonsmall cell lung cancer. Am J Med Sci 340:385–388. https://doi.org/10.1097/MAJ.0b013e3181e892a0
Ye L, Wang Y, Nie L et al (2017) MiR-130 exerts tumor suppressive function on the tumorigenesis of human non-small cell lung cancer by targeting PTEN. Am J Transl Res 9:1856–1865
Roman-Canal B, Moiola CP, Gatius S et al (2019) EV-associated miRNAs from pleural lavage as potential diagnostic biomarkers in lung cancer. Sci Rep 9:15057. https://doi.org/10.1038/s41598-019-51578-y
Tamiya H, Mitani A, Saito A et al (2018) Exosomal MicroRNA expression profiling in patients with lung adenocarcinoma-associated malignant pleural effusion. Anticancer Res 38:6707–6714. https://doi.org/10.21873/anticanres.13039
Singh M, Garg N, Venugopal C et al (2015) STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget 6:27461–27477. https://doi.org/10.18632/oncotarget.4742
Wei C-H, Wu G, Cai Q et al (2017a) MicroRNA-330-3p promotes cell invasion and metastasis in non-small cell lung cancer through GRIA3 by activating MAPK/ERK signaling pathway. J Hematol Oncol 10:125. https://doi.org/10.1186/s13045-017-0493-0
Sun G, Ding X, Bi N et al (2018) MiR-423-5p in brain metastasis: potential role in diagnostics and molecular biology. Cell Death Dis 9:936. https://doi.org/10.1038/s41419-018-0955-5
Li J, Feng Q, Wei X, Yu Y (2016) MicroRNA-490 regulates lung cancer metastasis by targeting poly r(C)-binding protein 1. Tumor Biol 37:15221–15228. https://doi.org/10.1007/s13277-016-5347-9
Arora S, Ranade AR, Tran NL et al (2011) MicroRNA-328 is associated with (non-small) cell lung cancer (NSCLC) brain metastasis and mediates NSCLC migration. Int J Cancer 129:2621–2631. https://doi.org/10.1002/ijc.25939
Chen LT, Xu SD, Xu H et al (2012) MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol 29:1673–1680. https://doi.org/10.1007/s12032-011-0083-x
Hwang SJ, Lee HW, Kim HR et al (2015) Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget 6:20434–20448. https://doi.org/10.18632/oncotarget.3886
Zhao C, Xu Y, Zhang Y et al (2013) Downregulation of miR-145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol Rep 30:2027–2034. https://doi.org/10.3892/or.2013.2728
Chen LJ, Li XY, Zhao YQ et al (2017) Down-regulated microRNA-375 expression as a predictive biomarker in non-small cell lung cancer brain metastasis and its prognostic significance. Pathol Res Pract 213:882–888. https://doi.org/10.1016/j.prp.2017.06.012
Wang FF, Wang S, Xue WH, Cheng JL (2016) microRNA-590 suppresses the tumorigenesis and invasiveness of non-small cell lung cancer cells by targeting ADAM9. Mol Cell Biochem 423:29–37. https://doi.org/10.1007/s11010-016-2822-y
He X, Chen S, Yang Z et al (2018) miR-4317 suppresses non-small cell lung cancer (NSCLC) by targeting fibroblast growth factor 9 (FGF9) and cyclin D2 (CCND2). J Exp Clin Cancer Res 37:230. https://doi.org/10.1186/s13046-018-0882-4
Guo Q, Zhang H, Zhang L et al (2015) MicroRNA-21 regulates non-small cell lung cancer cell proliferation by affecting cell apoptosis via COX-19. Int J Clin Exp Med 8:8835–8841
Wang X, Liu S, Zhou Z et al (2017b) A herpes simplex virus type 2-encoded microRNA promotes tumor cell metastasis by targeting suppressor of cytokine signaling 2 in lung cancer. Tumor Biol 39(5):1010428317701633. https://doi.org/10.1177/1010428317701633
Kuo PL, Liao SH, Hung JY et al (2013) MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim Biophys Acta Gen Subj 1830:3756–3766. https://doi.org/10.1016/j.bbagen.2013.02.022
Xu S, Yang F, Liu R et al (2018) Serum microRNA-139-5p is downregulated in lung cancer patients with lytic bone metastasis. Oncol Rep 39:2376–2384. https://doi.org/10.3892/or.2018.6316
Valencia K, Luis-Ravelo D, Bovy N et al (2014) MiRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 8:689–703. https://doi.org/10.1016/j.molonc.2014.01.012
Wei Y, Li D, Wang D et al (2017b) Evaluation of microRNA-203 in bone metastasis of patients with non-small cell lung cancer through TGF-β/SMAD2 expression. Oncol Rep. https://doi.org/10.3892/or.2017.5987
Hu X, Luo J (2018) Heterogeneity of tumor lymphangiogenesis: progress and prospects. Cancer Sci 109:3005–3012. https://doi.org/10.1111/cas.13738
Aguadé-Gorgorió G, Solé R (2019) Genetic instability as a driver for immune surveillance. J Immunother Cancer 7:345. https://doi.org/10.1186/s40425-019-0795-6
Mittal D, Gubin MM, Schreiber RD, Smyth MJ (2014) New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 27:16–25. https://doi.org/10.1016/j.coi.2014.01.004
Regan E, Sibley RC, Cenik BK et al (2016) Identification of gene expression differences between lymphangiogenic and non-lymphangiogenic non-small cell lung cancer cell lines. PLoS One 11(3):e0150963. https://doi.org/10.1371/journal.pone.0150963
Huang Q, Zhang Z, Liao Y et al (2018) 17β-estradiol upregulates IL6 expression through the ERβ pathway to promote lung adenocarcinoma progression. J Exp Clin Cancer Res 37:133. https://doi.org/10.1186/s13046-018-0804-5
Li Y, Ye Z, Chen S et al (2018b) ARHGEF19 interacts with BRAF to activate MAPK signaling during the tumorigenesis of non-small cell lung cancer. Int J Cancer 142:1379–1391. https://doi.org/10.1002/ijc.31169
Wu JI, Lin YP, Tseng CW et al (2019a) Crabp2 promotes metastasis of lung cancer cells via HuR and integrin β1/FAK/ERK signaling. Sci Rep 9:845. https://doi.org/10.1038/s41598-018-37443-4
Zhang N, Zhang SW (2019) Identification of differentially expressed genes between primary lung cancer and lymph node metastasis via bioinformatic analysis. Oncol Lett 18:3754–3768. https://doi.org/10.3892/ol.2019.10723
Zhao W, Wang J, Zhu B et al (2016) IGFBP7 functions as a potential lymphangiogenesis inducer in non-small cell lung carcinoma. Oncol Rep 35:1483–1492. https://doi.org/10.3892/or.2015.4516
Zhang ZF, Ma JQ, Zhang L (2005) Expression and clinical significance of MAPK in non-small cell lung cancer. Natl Med J China 85:339–342
Chuang HC, Chang CC, Teng CF et al (2019) MAP 4K3/GLk promotes lung cancer metastasis by phosphorylating and activating IQGAP1. Cancer Res 79:4978–4993. https://doi.org/10.1158/0008-5472.CAN-19-1402
Guo Y, Pan W, Liu S et al (2020) ERK/MAPK signalling pathway and tumorigenesis (Review). Exp Ther Med 19:1997–2007. https://doi.org/10.3892/etm.2020.8454
Hung P-S, Huang M-H, Kuo Y-Y, Yang JC-H (2020) The Inhibition of Wnt Restrain KRASG12V-Driven Metastasis in Non-Small-Cell Lung Cancer. Cancers (Basel) 12:837. https://doi.org/10.3390/cancers12040837
Wakamatsu N, Collins JB, Parker JS et al (2008) Gene expression studies demonstrate that the K-ras/Erk MAP kinase signal transduction pathway and other novel pathways contribute to the pathogenesis of cumene-induced lung tumors. Toxicol Pathol 36:743–752. https://doi.org/10.1177/0192623308320801
Du Rand I, Maskell N (2010) British Thoracic Society pleural disease guideline 2010. Br Thorac Soc 65:ii1–ii3. https://doi.org/10.1136/thx.2010.137042
Charalampidis C, Youroukou A, Lazaridis G et al (2015) Pleura space anatomy. J Thorac Dis 7:S27–S32. https://doi.org/10.3978/j.issn.2072-1439.2015.01.48
Donnenberg AD, Luketich JD, Dhupar R, Donnenberg VS (2019) Treatment of malignant pleural effusions: the case for localized immunotherapy. J Immunother Cancer 7:5–9. https://doi.org/10.1186/s40425-019-0590-4
Psallidas I, Kalomenidis I, Porcel JM et al (2016) Malignant pleural effusion: from bench to bedside. Eur Respir Rev 25:189–198. https://doi.org/10.1183/16000617.0019-2016
Semaan R, Feller-Kopman D, Slatore C et al (2016) Malignant pleural effusions. Am J Respir Crit Care Med 194:P11–P12. https://doi.org/10.1164/rccm.1946P11
Li T, Li H, Wang Y et al (2011) The expression of CXCR4, CXCL12 and CXCR7 in malignant pleural mesothelioma. J Pathol 223:519–530. https://doi.org/10.1002/path.2829
Liang JX, Gao W, Liang Y, Zhou XM (2015) Chemokine receptor CXCR4 expression and lung cancer prognosis: a meta-analysis. Int J Clin Exp Med 8:5163–5174
Oonakahara K, Matsuyama W, Higashimoto I et al (2004) Stromal-derived factor-1α/CXCL12-CXCR 4 axis is involved in the dissemination of NSCLC cells into pleural space. Am J Respir Cell Mol Biol 30:671–677. https://doi.org/10.1165/rcmb.2003-0340OC
Cavallaro S (2013) CXCR4/CXCL12 in non-small-cell lung cancer metastasis to the brain. Int J Mol Sci 14:1713–1727. https://doi.org/10.3390/ijms14011713
Guo F, Wang Y, Liu J et al (2016) CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene 35:816–826. https://doi.org/10.1038/onc.2015.139
Porcile C, Bajetto A, Barbieri F et al (2005) Stromal cell-derived factor-1α (SDF-1α/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res 308:241–253. https://doi.org/10.1016/j.yexcr.2005.04.024
Sigismund S, Avanzato D, Lanzetti L (2018) Emerging functions of the EGFR in cancer. Mol Oncol 12:3–20. https://doi.org/10.1002/1878-0261.12155
Zou JY, Bella AE, Chen ZG et al (2014) Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res 42:1110–1117. https://doi.org/10.1177/0300060514539273
Phillips RJ, Mestas J, Gharaee-Kermani M et al (2005) Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia ind. J Biol Chem 280:22473–22481. https://doi.org/10.1074/jbc.M500963200
Maulik G, Kijima T, Salgia R (2003) Role of receptor tyrosine kinases in lung cancer. In: Lung cancer. Humana Press, Totowa, NJ, pp 113–126
Wang H, Liu W, Wei D et al (2014) Effect of the LPA-mediated CXCL12-CXCR4 axis in the tumor proliferation, migration and invasion of ovarian cancer cell lines. Oncol Lett 7:1581–1585. https://doi.org/10.3892/ol.2014.1926
Shelton EL, Galindo CL, Williams CH et al (2013) Autotaxin signaling governs phenotypic heterogeneity in visceral and parietal mesothelia. PLoS One 8:e69712. https://doi.org/10.1371/journal.pone.0069712
Chu X, Wei X, Lu S, He P (2015) Autotaxin-LPA receptor axis in the pathogenesis of lung diseases. Int J Clin Exp Med 8:17117–17122
Ueda N, Minami K, Ishimoto K, Tsujiuchi T (2020) Effects of lysophosphatidic acid (LPA) receptor-2 (LPA2) and LPA3 on the regulation of chemoresistance to anticancer drug in lung cancer cells. Cell Signal 69:109551. https://doi.org/10.1016/j.cellsig.2020.109551
Houben AJS, Moolenaar WH (2011) Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 30:557–565. https://doi.org/10.1007/s10555-011-9319-7
Ren Y, Dai C, Zheng H et al (2016) Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 7:53245–53253. https://doi.org/10.18632/oncotarget.10644
Shiroyama T, Suzuki H, Tamiya M et al (2018) Clinical characteristics of liver metastasis in nivolumabtreated patients with non-small cell lung cancer. Anticancer Res 38:4723–4729. https://doi.org/10.21873/anticanres.12779
Xu Z, Yang Q, Chen X et al (2019) Clinical associations and prognostic value of site-specific metastases in non-small cell lung cancer: a population-based study. Oncol Lett 17:5590–5600. https://doi.org/10.3892/ol.2019.10225
Kagohashi K, Satoh H, Ishikawa H et al (2003) Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol 20:25–28. https://doi.org/10.1385/MO:20:1:25
Mishima S, Nozaki Y, Mikami S et al (2015) Diffuse liver metastasis of small-cell lung cancer presenting as acute liver failure and diagnosed by transjugular liver biopsy: a rare case in whom nodular lesions were detected by enhanced CT examination. Case Rep Gastroenterol 9:81–87. https://doi.org/10.1159/000381140
Riihimäki M, Hemminki A, Fallah M et al (2014) Metastatic sites and survival in lung cancer. Lung Cancer 86:78–84. https://doi.org/10.1016/j.lungcan.2014.07.020
Nakazawa K, Kurishima K, Tamura T et al (2012) Specific organ metastases and survival in small cell lung cancer. Oncol Lett 4:617–620. https://doi.org/10.3892/ol.2012.792
Doebele RC, Lu X, Sumey C et al (2012) Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 118:4502–4511. https://doi.org/10.1002/cncr.27409
Kuijpers CCHJ, Hendriks LEL, Derks JL et al (2018) Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer 121:76–81. https://doi.org/10.1016/j.lungcan.2018.05.006
Golding B, Luu A, Jones R, Viloria-Petit AM (2018) The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer 17:1–15. https://doi.org/10.1186/s12943-018-0810-4
Murray PB, Lax I, Reshetnyak A et al (2015) Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal 8:1–8. https://doi.org/10.1126/scisignal.2005916
Sakagami H, Hashimoto KEN, Suzuki F et al (2008) Tumor-specificity and type of cell death induced by vitamin K2 derivatives and prenylalcohols. Anticancer Res 158:151–158
Voena C, Varesio LM, Zhang L et al (2016) Oncogenic ALK regulates EMT in non-small cell lung carcinoma through repression of the epithelial splicing regulatory protein 1. Oncotarget 7:33316–33330. https://doi.org/10.18632/oncotarget.8955
An R, Wang Y, Voeller D et al (2016) CRKL mediates EML4-ALK signaling and is a potential therapeutic target for ALK-rearranged lung adenocarcinoma. Oncotarget 7:29199–29210. https://doi.org/10.18632/oncotarget.8638
Cooke M, Baker MJ, Kazanietz MG (2020) Rac-GEF/Rac Signaling and Metastatic Dissemination in Lung Cancer. Front Cell Dev Biol 8:1–7. https://doi.org/10.3389/fcell.2020.00118
Schmid S, Gautschi O, Rothschild S et al (2017) Clinical outcome of ALK-Positive Non–Small Cell Lung Cancer (NSCLC) patients with de novo EGFR or KRAS co-mutations receiving tyrosine kinase inhibitors (TKIs). J Thorac Oncol 12:681–688. https://doi.org/10.1016/j.jtho.2016.12.003
Marino FZ, Ronchi A, Accardo M, Franco R (2017) Concomitant ALK/KRAS and ALK/EGFR mutations in non small cell lung cancer: different profile of response to target therapies. Transl Cancer Res 6:S457–S460. https://doi.org/10.21037/tcr.2017.03.77
Sweis RF, Thomas S, Bank B et al (2016) Concurrent EGFR mutation and ALK translocation in non-small cell lung cancer. Cureus 8. https://doi.org/10.7759/cureus.513
Gonzales CB, De La Chapa JJ, Saikumar P et al (2016) Co-targeting ALK and EGFR parallel signaling in oral squamous cell carcinoma. Oral Oncol 59:12–19. https://doi.org/10.1016/j.oraloncology.2016.05.007
Miyawaki M, Yasuda H, Tani T et al (2017) Overcoming EGFR bypass signal-induced acquired resistance to ALK tyrosine kinase inhibitors in ALK-translocated lung cancer. Mol Cancer Res 15:106–114. https://doi.org/10.1158/1541-7786.MCR-16-0211
Meng DF, Xie P, Peng LX et al (2017) CDC42-interacting protein 4 promotes metastasis of nasopharyngeal carcinoma by mediating invadopodia formation and activating EGFR signaling. J Exp Clin Cancer Res 36:21. https://doi.org/10.1186/s13046-016-0483-z
Tonucci FM, Almada E, Borini-Etichetti C et al (2019) Identification of a CIP4 PKA phosphorylation site involved in the regulation of cancer cell invasiveness and metastasis. Cancer Lett 461:65–77. https://doi.org/10.1016/j.canlet.2019.07.006
Truesdell P, Ahn J, Chander H et al (2015) CIP4 promotes lung adenocarcinoma metastasis and is associated with poor prognosis. Oncogene 34:3527–3535. https://doi.org/10.1038/onc.2014.280
Gaspar LE (2004) Brain metastases in lung cancer. Expert Rev Anticancer Ther 4:259–270. https://doi.org/10.1586/14737140.4.2.259
Mujoomdar A, Austin JHM, Malhotra R et al (2007) Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 242:882–888. https://doi.org/10.1148/radiol.2423051707
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology 14:1171–1177. https://doi.org/10.1093/neuonc/nos152
Valiente M, Obenauf AC, Jin X et al (2014) Serpins promote cancer cell survival and vascular Co-option in brain metastasis. Cell 156:1002–1016. https://doi.org/10.1016/j.cell.2014.01.040
Kim SW, Choi HJ, Lee HJ et al (2014) Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro-Oncology 16:1585–1598. https://doi.org/10.1093/neuonc/nou128
Chen Q, Boire A, Jin X et al (2016) Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533:493–498. https://doi.org/10.1038/nature18268
Ellenbroek SIJ, Collard JG (2007) Rho GTPases: functions and association with cancer. Clin Exp Metastasis 24:657–672. https://doi.org/10.1007/s10585-007-9119-1
Masre SF, Rath N, Olson MF, Greenhalgh DA (2017) ROCK2/rasHa co-operation induces malignant conversion via p53 loss, elevated NF-κB and tenascin C-associated rigidity, but p21 inhibits ROCK2/NF-κB-mediated progression. Oncogene 36:2529–2542. https://doi.org/10.1038/onc.2016.402
Masre SF, Rath N, Olson MF, Greenhalgh DA (2020) Epidermal ROCK2 induces AKT1/GSK3β/β-catenin, NFκB and dermal tenascin C; but enhanced differentiation and p53/p21 inhibit papilloma. Carcinogenesis. https://doi.org/10.1093/carcin/bgz205
Wojciak-Stothard B, Ridley AJ (2002) Rho GTPases and the regulation of endothelial permeability. Vasc Pharmacol 39:187–199. https://doi.org/10.1016/S1537-1891(03)00008-9
Abbott NJ, Patabendige AAK, Dolman DEM et al (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Hanibuchi M, Kim SJ, Fidler IJ, Nishioka Y (2014) The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Investig 61:241–253
Li B, Zhao WD, Tan ZM et al (2006) Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett 580:4252–4260. https://doi.org/10.1016/j.febslet.2006.06.056
Maekawa M, Ishizaki T, Boku S et al (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285:895–898. https://doi.org/10.1126/science.285.5429.895
Chang YC, Stins MF, McCaffery MJ et al (2004) Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun 72:4985–4995. https://doi.org/10.1128/IAI.72.9.4985-4995.2004
Lee TH, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278:5277–5284. https://doi.org/10.1074/jbc.M210063200
Li B, Wang C, Zhang Y et al (2013a) Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene 32:2952–2962. https://doi.org/10.1038/onc.2012.313
Hauck CR, Thibodeau BJ, Ahmed S et al (2017) Targeted DNA sequencing of non–small cell lung cancer identifies mutations associated with brain metastases. Int J Radiat Oncol 99:S199–S200. https://doi.org/10.1016/j.ijrobp.2017.06.495
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. https://doi.org/10.1038/nrg1879
Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci 22:267–272. https://doi.org/10.1016/S0968-0004(97)01061-X
Gray JW (2016) PI3 kinase pathway mutations in human cancers. JAMA Oncol 2:1543–1544. https://doi.org/10.1001/jamaoncol.2016.0875
Steelman LS, Stadelman KM, Chappell WH et al (2008) Akt as a therapeutic target in cancer. Expert Opin Ther Targets 12:1139–1165. https://doi.org/10.1517/14728222.12.9.1139
Tian X, Zhou D, Chen L et al (2018) Polo-like kinase 4 mediates epithelial–mesenchymal transition in neuroblastoma via PI3K/Akt signaling pathway. Cell Death Dis 9:54. https://doi.org/10.1038/s41419-017-0088-2
David O, Jett J, LeBeau H et al (2004) Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res 10:6865–6871. https://doi.org/10.1158/1078-0432.CCR-04-0174
Jin Y, Yuan Y, Yi M et al (2019) Phosphorylated-Akt overexpression is associated with a higher risk of brain metastasis in patients with non-small cell lung cancer. Biochem Biophys Rep 18:100625. https://doi.org/10.1016/j.bbrep.2019.100625
Li Q, Yang J, Yu Q et al (2013b) Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clin Cancer Res 19:6252–6260. https://doi.org/10.1158/1078-0432.CCR-13-1093
da Silva GT, Bergmann A, Thuler LCS (2019) Incidence and risk factors for bone metastasis in Non-Small Cell Lung Cancer. Asian Pacific J Cancer Prev 20:45–51. https://doi.org/10.31557/APJCP.2019.20.1.45
Kuchuk M, Kuchuk I, Sabri E et al (2015) The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer 89:197–202. https://doi.org/10.1016/j.lungcan.2015.04.007
Decroisette C, Monnet I, Berard H et al (2011) Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol 6:576–582. https://doi.org/10.1097/JTO.0b013e318206a1e3
Kakhki VRD, Anvari K, Sadeghi R et al (2013) Pattern and distribution of bone metastases in common malignant tumors. Nucl Med Rev 16:66–69. https://doi.org/10.5603/NMR.2013.0037
Andrade K, Fornetti J, Zhao L et al (2017) RON kinase: a target for treatment of cancer-induced bone destruction and osteoporosis. Sci Transl Med 9:eaai9338. https://doi.org/10.1126/scitranslmed.aai9338
Fazzalari NL (2008) Bone remodeling: a review of the bone microenvironment perspective for fragility fracture (osteoporosis) of the hip. Semin Cell Dev Biol 19:467–472. https://doi.org/10.1016/j.semcdb.2008.08.003
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94:25–34. https://doi.org/10.1007/s00223-013-9774-y
Roodman GD (2004) Mechanisms of Bone Metastasis. N Engl J Med 350:1655–1664. https://doi.org/10.1056/NEJMra030831
Virk MS, Lieberman JR (2007) Tumor metastasis to bone. Arthritis Res Ther 9:1–10. https://doi.org/10.1186/ar2169
Stern PH, Tatrai A, Semler DE et al (1995) Endothelin receptors, second messengers, and actions in bone. J Nutr 125:2028S–2032S. https://doi.org/10.1093/jn/125.suppl_7.2028S
Hall CL, Kang S, MacDougald OA, Keller ET (2006) Role of WNTS in prostate cancer bone metastases. J Cell Biochem 97:661–672. https://doi.org/10.1002/jcb.20735
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055. https://doi.org/10.1210/endo.142.12.8536
Kang Y, Siegel PM, Shu W et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549. https://doi.org/10.1016/S1535-6108(03)00132-6
Shariat SF, Andrews B, Kattan MW et al (2001) Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58:1008–1015. https://doi.org/10.1016/S0090-4295(01)01405-4
Bobinac D, Marić I, Zoričić S et al (2005) Expression of bone morphogenetic proteins in human metastatic prostate and breast cancer. Croat Med J 46:389–396
Sottnik JL, Dai J, Zhang H et al (2015) Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res 75:2151–2158. https://doi.org/10.1158/0008-5472.CAN-14-2493
Krzeszinski JY, Wan Y (2015) New therapeutic targets for cancer bone metastasis. Trends Pharmacol Sci 36:360–373. https://doi.org/10.1016/j.tips.2015.04.006
Luo Q, Xu Z, Wang L et al (2016) Progress in the research on the mechanism of bone metastasis in lung cancer. Mol Clin Oncol 5:227–235. https://doi.org/10.3892/mco.2016.917
D’Antonio C, Passaro A, Gori B et al (2014) Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 6:101–114. https://doi.org/10.1177/1758834014521110
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S (2020) RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int 2020:1–11. https://doi.org/10.1155/2020/6910312
Miki T, Yano S, Hanibuchi M et al (2004) Parathyroid hormone-related protein (PTHRP) is responsible for production of bone metastasis, but not visceral metastasis, by human small cell lung cancer SBC-5 cells in natural killer cell-depleted scid mice. Int J Cancer 108:511–515. https://doi.org/10.1002/ijc.11586
Roato I (2014) Bone metastases: when and how lung cancer interacts with bone. World J Clin Oncol 5:149–155
Ell B, Mercatali L, Ibrahim T et al (2013) Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24:542–556. https://doi.org/10.1016/j.ccr.2013.09.008
Karapanagiotou EM, Terpos E, Dilana KD et al (2010) Serum bone turnover markers may be involved in the metastatic potential of lung cancer patients. Med Oncol 27:332–338. https://doi.org/10.1007/s12032-009-9214-z
Peng X, Guo W, Ren T et al (2013) Differential expression of the RANKL/RANK/OPG system is associated with bone metastasis in human non-small cell lung cancer. PLoS One 8:e58361. https://doi.org/10.1371/journal.pone.0058361
Zhu X, Luo H, Xu Y (2019) Transcriptome analysis reveals an important candidate gene involved in both nodal metastasis and prognosis in lung adenocarcinoma. Cell Biosci 9:82. https://doi.org/10.1186/s13578-019-0356-1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aminuddin, A., Masre, S.F., Teow, SY., Ng, P.Y. (2021). Exploring the ‘Dormancy Activation Switch’ in the Tumour Microenvironment for Metastatic Lung Cancer: The Possible Role of MicroRNA. In: Dua, K., Löbenberg, R., Malheiros Luzo, Â.C., Shukla, S., Satija, S. (eds) Targeting Cellular Signalling Pathways in Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-33-6827-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-33-6827-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6826-2
Online ISBN: 978-981-33-6827-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)