Abstract
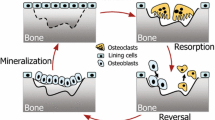
Minimodeling is modeling on a microscopic level and occurs on trabecular, endocortical, and periosteal surfaces. Precursor cells are activated to osteoblasts that form new bone, called formation modeling. When osteoclastic precursor cells are activated and osteoclasts resorb bone, it is called resorption modeling. These processes are influenced by mechanical loading at physiological or supra-physiological force levels, and by various metabolic bone diseases and drugs for the treatment of osteoporosis.
The chapter illustrates two drift patterns in elongation of long tubular bone (growth) and healing of angulated tubular bone to be straight in children. The latter occurs under the influence of supra-physiologic mechanical loading, that is compression force on the cortex of the concave side, and tensile force on the convex side. Minimodeling is observed in dialysis patients with adynamic bone disease. Vitamin D and its derivative and human PTH(1–34) stimulate minimodeling in cancellous bone.
In the last decade, several drugs have been developed and are available for the treatment of osteoporotic patients. Therapeutic effects of each drug have a different proportion of action on remodeling and modeling, which cannot be identified by DXA. There has been renewed interest lately in the role of “minimodeling,” that is modeling-based formation (MBF) during osteoporosis therapy.
Recent reports of early effects of an established anabolic (teriparatide) versus antiresorptive (denosumab) agent were described on three bone envelopes: cancellous, periosteal, and endocortical surfaces in human transiliac bone biopsies. Renamed terms on bone formation were defined and described.
The present invited review was completed and submitted to the publisher on 23-Sep-20.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Milch RA, Rall DP, Tobie JE. Bone localization of the tetracycline. J Natl Cancer Inst. 1957;19:87.
Frost HM. Preparation of thin, undecalcified bone sections by rapid manual method. Stain Tech. 1958;33:273–6.
Frost HM. Staining of fresh, undecalcified thin bone sections. Stain Tech. 1959;34:135–46.
Frost HM, Roth H, Villanueva AR, Stanisavljevic S. Experimental multiband tetracycline measurement of lamellar osteoblastic activity. Henry Ford Hosp Med Bull. 1961;9:312–29.
Frost HM. Bone remodeling dynamics. Springfield, IL: Charles C Thomas; 1963.
Frost HM. Mathematical elements of lamellar bone remodeling. Springfield, IL: Charles C Thomas; 1964.
Frost HM. Tetracycline-based histological analysis of bone remodeling. Calc Tiss Res. 1969;3:211–37.
Recker RR, editor. Bone histomorphometry: techniques and interpretation. Boca Raton, FL: CRC Press; 1983.
Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610.
Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17.
Frost HM. Physiology of bone, cartilage and fibrous tissue. Springfield: Charles C Thomas; 1972.
Allen MR, Burr DB. Bone growth, modeling and remodeling. In: Burr DB, Allen MR, editors. Basic and applied bone biology. 2nd ed. Academic; 2019. p. 85–100.
Takahashi H, Epker B, Frost HM. Resorption precedes formative activity. Surg Forum. 1964;15:437–8.
Takahashi H, Hattner R, Epker B, Frost HM. Evidence that Bone resorption precedes formation at the cellular level. Henry Ford Hosp Med Bull. 1964;12:359–64.
Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodeling. Nature. 1965;206(983):489–90.
Baron R. Importance of the intermediate phases between resorption and formation in the measurement and understanding of the bone remodeling sequence. In: Meunier PJ, editor. Bone histomorphometry: second international workshop Lyon. Toulouse: Armour Montagu; 1977. p. 179–83.
Parfitt AM: The cellular basis of bone remodeling: The quantum concept reviewed in the light of recent advances in the cell biology of bone. Calc Tiss Int 1984;Suppl 36: 37–45.
Delaisse JM. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014;3:561.
Andersen TL, Abdelgawad ME, Kristensen HB, et al. Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol. 2013;183(1):235–46. https://doi.org/10.1016/j.ajpath.2013.03.006.
Frost HM. Strains and other mechanical influences on bone strength and maintenance. Curr Opin Orthop. 1997;8:60–70.
Frost HM. The Utah paradigm of skeletal physiology. Vol. I, International Society of Musculoskeletal and Neuronal Interactions; 2002.
Chow JWM, Badve S, Chambers TJ. Bone formation is not coupled to bone resorption in site – specific manner in adults rats. Anat Rec. 1993;236:366–72.
Erben RG. Trabecular and endocortical bone surfaces in the rat: modeling or remodeling? Anat Rec. 1996;246(1):39–46.
Kobayashi S, Takahashi HE, Ito A, et al. Trabecular minimodeling in human iliac bone. Bone. 2003;32(2):163–9. https://doi.org/10.1016/s8756-3282(02)00947-x.
Ubara Y, Fushimi T, Tagami T, et al. Histomorphometric features of bone in patients with primary and secondary hypoparathyroidism. Kidney Int. 2003;63(5):1809–16.
Ubara Y, Tagami T, Nakanishi S, et al. Significance of minimodeling in dialysis patients with adynamic bone disease. Kidney Int. 2005;68(2):833–9. https://doi.org/10.1111/j.1523-1755.2005.00464.x.
Yajima A, Inaba M, Tominaga Y, et al. Minimodeling reduces the rate of cortical bone loss in patients with secondary hyperparathroidim. Am J Kidney Dis. 2007;49(3):440–51.
Erben RG, Weiser H, Sinowatz F, et al. Vitamin D metabolites prevent vertebral osteopenia in ovariectomized rats. Calcif Tissue Int. 1992;50:228–36.
Li M, Healy DR, Li Y, et al. Alfacalcidol prevents age-related bone loss and causes an atypical pattern of bone formation in aged male rats. J Musculoskelet Neuronal Interact. 2004;4(1):22–32.
Liu XQ, Chen HY, Tian XY, et al. Alfacalcidol treatment increases bone mass from anticatabolic and anabolic effects on cancellous and cortical bone in intact female rats. J Bone Miner Metab. 2008;26:425–35.
de Freitas PHL, Hasegawa T, Amizuka N, et al. Eldecalcitol, a second-generation vitamin D analog, drives bone minimodeling and reduces osteoclastic number in trabecular bone of ovariectomized rats. Bone. 2011;49(3):335–42.
Saito H, Takeda S, Amizuka N. Eldecalcitol and calcitoriol stimulates ‘bone minimodeling’, focal bone formation without prior bone resorption, in rat trabecular bone. J Steroid Biochem Mol Biol. 2013;136:178–82.
Hikata T, Hasegawa T, Horiuchi K, et al. Histomorphometric analysis of minimodeling in the vertebrae in postmenopausal patients treated with anti-osteoporotic agents. Bone Rep. 2016;5:286–91.
Inoue J. Bone changes with long term administration of low dose 1-34 human PTH on adult beagles. J Jpn Orthop Ass. 1985;59:409–27.
Li M, Liang H, Shen Y, et al. Parathyroid hormone stimulates cancellous bone formation at skeletal sites regardless of marrow composition in ovariectomized rats. Bone. 1999;24:95–100.
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18(1):18–23. https://doi.org/10.1359/jbmr.2003.18.1.18.
Ma YL, Zeng Q, Donley DW, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855–64.
Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366–73.
Jee WS, Tian XY, Setterberg RB. Cancellous bone minimodeling-based formation: a Frost, Takahashi legacy. J Musculoskelet Neuronal Interact. 2007;7(3):232–9.
Dobnig H, Stepan JJ, Burr DB, et al. Teriparatide reduces bone microdamage accumulation in postmenopausal women previously treated with alendronate. J Bone Miner Res. 2009;24(12):1998–2006.
Lindsay R, Zhou H, Cosman F, et al. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2009;22(4):495–502.
Nakamura T, Sugimoto T, Nakano T, et al. Randomized teriparatide [human parathyroid hormone (PTH)1–34] once-weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97:3097–106.
Sugimoto T, Shiraki M, Fukunaga M, et al. Study of twice-weekly injections of Teriparatide by comparing efficacy with once-weekly injections in osteoporosis patients: the TWICE study. Osteoporosis Int. 2019;30:2321–31. doi.org/10.1007/s00198-019-05111-6
Dempster DW, Zhou H, Recker RR, et al. Remodeling- and modeling-based bone formation with teriparatide versus denosumab: a longitudinal analysis from baseline to 3 months in the AVA Study. J Bone Miner Res. 2017;33(2):298–306. https://doi.org/10.1002/jbmr.3309.
Bone HG, Wagman RB, Brandi ML, et al. 10 years of Denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomized FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–23. https://doi.org/10.1016/S2213-8587(17)30138-9.
Dempster DW, Chines A, Bostrom MP, et al. Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J Bone Miner Res. 2020;35(7):1282–8. https://doi.org/10.1002/jbmr.4006.
Eriksen EF, Chapurlat R, Brown JP, et al.: Extensive modeling-based bone formation after 2 months of romosozumab treatemnt: results from the FRAME Clinical Trial. Annual Meeting ASBMF 2019, Abstract 1049.
Dempster DW, Nieves J, Zhou H, et al. Effects of Teriparatide on modeling-based and remodeling-based bone formation in the human femoral neck. Annual meeting of American Society for Bone Mineral Research, Sept, 12, 2020, Abstract 1039.
Dempster DW, Zhou H, Rao SD, et al.: Effects of abaloparatide on modeling and remodeling based bone formation. Annual meeting of American Society for Bone Mineral Research, Sept, 12, 2020, Abstract 1040.
Sano H, Kondo N, Shimakura T, et al. Evidence for ongoing modeling-based bone formation in human femoral head trabeculae via forming minimodeling structure: a study in patients with fracture and arthritis. Front Endocrinol. 2018;9:88. https://doi.org/10.3389/fendo.2018.00088.
Villanueva AR, Kundin KD. A veratile new mineralized bone stain for simultaneous assessment of tetracycline and osteoid seams. Stain Technol. 1989;64:129–38.
Villanueva AR. Preparation and staining of mineralized sections of bone. In: Takahashi HE, editor. Handbook of bone morphometry. 2nd ed. Niigata: Nishimura Publisher; 1997. p. 27–40.
Acknowledgments
Authors are most grateful to Prof. David W. Dempster in reviewing the manuscript for his critical and constructive comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Takahashi, H.E., Yamamoto, N., Sano, H., Shimakura, T. (2022). Bone Minimodeling, Modeling-Based Bone Formation in Trabecular, Endocortical and Periosteal Bone. In: Takahashi, H.E., Burr, D.B., Yamamoto, N. (eds) Osteoporotic Fracture and Systemic Skeletal Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-5613-2_5
Download citation
DOI: https://doi.org/10.1007/978-981-16-5613-2_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5612-5
Online ISBN: 978-981-16-5613-2
eBook Packages: MedicineMedicine (R0)