Abstract
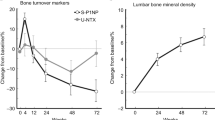
Development of novel therapies for bone diseases can benefit from mathematical models that predict drug effect on bone remodeling biomarkers. Therefore, a bone cycle model (BCM) was developed that takes into consideration the concept of the basic multicellular unit and the dynamic equilibrium of bone remodeling. The model is a closed form cyclical model with four compartments representing resorption, formation, primary mineralization, and secondary mineralization. Equations describing the time course of bone turnover biomarkers were developed using the flow rate of bone cycle units (BCU) between the compartments or the amount of BCU in each compartment. A disease progression model representing bone loss in osteoporosis, a vitamin D and calcium supplementation (placebo) model, and a drug model for antiresorptive treatments were added to the model. Initial model parameter values were derived from published bone turnover data. The BCM accurately described biomarker-time profiles in postmenopausal women receiving either placebo or bisphosphonate treatment. The slow continual increase in bone mineral density (BMD) observed after 1 year of treatment was accurately described when changes in bone turnover were combined with increases in mineralization. For this purpose, the secondary mineralization compartment was replaced by three catenary chain compartments representing increasing mineral content. The refined BCM satisfactorily predicted biomarker profiles after long-term (10-year) bisphosphonate treatment. Furthermore, the model successfully described individual bone turnover markers and BMD results following treatment with denosumab in postmenopausal women. Analyses with this model could be used to optimize dosing regimens and to predict effects of novel osteoporotic treatments.









Similar content being viewed by others
References
Parfitt AM (1994) Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 55:273–286
Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD (2000) A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res 15:557–563
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Hernandez CJ, Beaupre GS, Marcus R, Carter DR (2001) A theoretical analysis of the contributions of remodeling space, mineralization, and bone balance to changes in bone mineral density during alendronate treatment. Bone 29:511–516
Hazelwood SJ, Bruce Martin R, Rashid MM, Rodrigo JJ (2001) A mechanistic model for internal bone remodeling exhibits different dynamic responses in disuse and overload. J Biomech 34:299–308
Nyman JS, Yeh OC, Hazelwood SJ, Martin RB (2004) A theoretical analysis of long-term bisphosphonate effects on trabecular bone volume and microdamage. Bone 35:296–305
Heaney RP (1994) The bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res 9:1515–1523
Tayyar S, Weinhold PS, Butler RA, Woodard JC, Zardiackas LD, St John KR, Bledsoe JM, Gilbert JA (1999) Computer simulation of trabecular remodeling using a simplified structural model. Bone 25:733–739
Heaney RP, Yates AJ, Santora AC 2nd (1997) Bisphosphonate effects and the bone remodeling transient. J Bone Miner Res 12:1143–1151
Hernandez CJ, Beaupre GS, Marcus R, Carter DR (2002) Long-term predictions of the therapeutic equivalence of daily and less than daily alendronate dosing. J Bone Miner Res 17:1662–1666
Martin MJ, Buckland-Wright JC (2004) Sensitivity analysis of a novel mathematical model identifies factors determining bone resorption rates. Bone 35:918–928
Pharsight Trial Simulator User’s Guide (version 2.2). Pharsight Corporation, Mountain View, CA
Beal S, Sheiner LB, Boeckmann A, Bauer RJ (2009) NONMEM User’s Guides, 1989-2009. Icon Development Solutions, Ellicott City, MD
S-PLUS 6.2 Modern Statistics and Advanced Graphics. Insightful Corporation, Seattle, WA
Development Core Team (2007) A language and environment for statistical computing. Foundation for Statistical Computing, Vienna
Post TM, Cremers SC, Kerbusch T, Danhof M (2010) Bone physiology, disease and treatment: towards disease system analysis in osteoporosis. Clin Pharmacokinet 49:89–118
Berne RM, Levy MN (1998) Endocrine regulation of calcium and phosphate metabolism. In: Principles of physiology. Elsevier, New York, pp 850–871
Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tanko LB (2006) An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol 62:781–792
Compston J (2006) Bone quality: what is it and how is it measured? Arq Bras Endocrinol Metabol 50:579–585
Hernandez CJ, Beaupre GS, Keller TS, Carter DR (2001) The influence of bone volume fraction and ash fraction on bone strength and modulus. Bone 29:74–78
Hernandez CJ (2008) How can bone turnover modify bone strength independent of bone mass? Bone 42:1014–1020
Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ (2004) Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol 229:293–309
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C (2003) Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone 33:301–307
Rosen CJ, Khosla S (2010) Placebo-controlled trials in osteoporosis–proceeding with caution; discussion e22. N Engl J Med 363:1365–1367
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337:670–676
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683
Gieschke R, Hayashi N, Vis P, Jacqmin P (2003) Modelling the effect of placebo and calcium and vitamin D supplementation on the time course of biomarkers of bone turnover in osteoporotic postmenopausal women. Poster presented at: 30th European Symposium on Calcified Tissues; 8–12 May; Rome, Italy. Poster 280
Zheng J, van Schaick E, Wu LS, Jacqmin P, Perez Ruixo JJ (2015) Using early biomarker data to predict long-term bone mineral density: application of semi-mechanistic bone cycle model on denosumab data. Accepted, J Pharmacokinet Pharmacodyn
Russell RG, Croucher PI, Rogers MJ (1999) Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int 9(Suppl 2):S66–S80
Ravn P, Christensen JO, Baumann M, Clemmesen B (1998) Changes in biochemical markers and bone mass after withdrawal of ibandronate treatment: prediction of bone mass changes during treatment. Bone 22:559–564
Thiebaud D, Burckhardt P, Kriegbaum H, Huss H, Mulder H, Juttmann JR, Schoter KH (1997) Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med 103:298–307
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Pillai G, Gieschke R, Goggin T, Jacqmin P, Schimmer RC, Steimer JL (2004) A semimechanistic and mechanistic population PK-PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol 58:618–631
Cremers S, Sparidans R, den Hartigh J, den Hartigh J, Hamdy N, Vermeij P, Papapoulos S (2002) A pharmacokinetic and pharmacodynamic model for intravenous bisphosphonate (pamidronate) in osteoporosis. Eur J Clin Pharmacol 57:883–890
Jacqmin P, Snoeck E, van Schaick EA, Gieschke R, Pillai P, Steimer JL, Girard P (2007) Modelling response time profiles in the absence of drug concentrations: definition and performance evaluation of the K-PD model. J Pharmacokinet Pharmacodyn 34:57–85
Gabrielsson J, Jusko WJ, Alari L (2000) Modeling of dose-response-time data: four examples of estimating the turnover parameters and generating kinetic functions from response profiles. Biopharm Drug Dispos 21:41–52
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Chesnut IC, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD (2000) Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 85:3109–3115
Lin JH (1996) Bisphosphonates: a review of their pharmacokinetic properties. Bone 18:75–85
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, Martin JS, Amg Bone Loss Study Group (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Holford NH, Kimko HC, Monteleone JP, Peck CC (2000) Simulation of clinical trials. Annu Rev Pharmacol Toxicol 40:209–234
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R, Resch H, Verbruggen N, Hustad CM, DaSilva C, Petrovic R, Santora AC, Ince BA, Lombardi A (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Gallagher JC, Rapuri PB, Haynatzki G, Detter JR (2002) Effect of discontinuation of estrogen, calcitriol, and the combination of both on bone density and bone markers. J Clin Endocrinol Metab 87:4914–4923
Marathe DD, Marathe A, Mager DE (2011) Integrated model for denosumab and ibandronate pharmacodynamics in postmenopausal women. Biopharm Drug Dispos 32:471–481
Schmidt S, Post TM, Peletier LA, Boroujerdi MA, Danhof M (2011) Coping with time scales in disease systems analysis: application to bone remodeling. J Pharmacokinet Pharmacodyn 38:873–900
Post TM, Schmidt S, Peletier LA, de Greef R, Kerbusch T, Danhof M (2013) Application of a mechanism-based disease systems model for osteoporosis to clinical data. J Pharmacokinet Pharmacodyn 40:143–156
European Medicines Agency (2005) Guideline on the evaluation of new medicinal products in the treatment of primary osteoporosis. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003406.pdf. Accessed 1 Nov 2012
Food and Drug Administration (1994) Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis, Division of Metabolic and Endocrine Drug Products. http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM131206.pdf. Accessed 1 Nov 2012
Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, Chow AT, Perez-Ruixo JJ (2011) Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet 50:793–807
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536
Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87:1586–1592
Hollister S (2004) BME 456: Biosolid Mechanics: Modeling and Applications http://www.engin.umich.edu/class/bme456/bonestructure/bonestructure.htm. Accessed 1 Nov 2012
Melsen F, Mosekilde L (1980) Trabecular bone mineralization lag time determined by tetracycline double-labeling in normal and certain pathological conditions. Acta Pathol Microbiol Scand A 88:83–88
Ganong WF (1995) Hormonal control of calcium metabolism and the physiology of bone. Review of medical physiology, 17th edn. Simon and Schuster, New York. pp 352–364
Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC (2000) Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab 85:3011–3019
Acknowledgments
Amgen Inc funded this study. We thank Janet R Wade, PhD, (SGS Exprimo NV) for her editorial suggestions. Lisa A Humphries, PhD, of Amgen Inc provided editing and formatting support.
Conflict of interest
Amgen Inc sponsored this study and was involved in the study design, data collection, analysis, interpretation, writing of the manuscript, and the decision to submit the manuscript for publication. EVS and PJ consult for Amgen Inc; JJPR is employed by Amgen Inc and has Amgen Inc stock and/or stock options; JZ was employed by Amgen Inc and had Amgen Inc stock and/or stock options at the time of manuscript preparation; JZ is currently employed by Pfizer; RG is employed by F Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Appendices
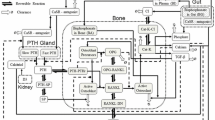
Appendix 1: Basic bone cycle model
Model assumptions
-
Lifespan can be converted to a first-order rate constant.
-
Origination frequency is proportional to BCUs in the mineralization compartments.
-
Bone from both fast and slow mineralization stages can be resorbed.
-
Fast and slow mineralization contributes to the same extent to BMD.
-
No mineralization lag-time.
-
Slow mineralization has a duration of approximately 3 years.
-
BMD at one specific site (e.g. lumbar spine) can be correlated to the overall changes of BTM in plasma such as CTX or osteocalcin.
-
There is no seasonal fluctuation in bone remodeling.
Total amount of BCU is based on a distribution of the BCU over the various compartments
Model equations of the various compartments
Baseline or steady state conditions
Lifespan parameters are transferred to first-order rate constants
k SM = 1/lifespan of BCU in slow mineralization process, k RC = 1/life span of BCU in resorption cavities, k CF = 1/life span of BCU in matrix formation and k FM = 1/lifespan of BCU in fast mineralization phase.
The biomarker CTX is assumed to be determined by the amount of BCU in the mineralization compartments and the rate of transfer of the BCUs from the mineralization compartment to the resorption compartment.
where BCU FM and BCU SM represent the amount of BCUs in the fast and slow mineralization compartments, and the parameters BCU FM,0 and BCU SM,0 represent the amounts of BCU at baseline. In this way, the CTX parameters are dimensionless and described as percentage change from baseline.
For the biomarker osteocalcin, the output rate of BCUs from the collagen formation compartment was used, as this biomarker is exclusively released by osteoblasts and is deposited into bone matrix. Since osteocalcin had an apparent residual concentration not related to bone turnover, an empirical correction factor of 1.44 was used.
For BMD, the amounts of BCUs in the fast and slow mineralization compartments were used.
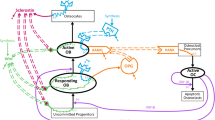
Appendix 2: Final bone cycle model (Fig. 6)
Model assumptions
-
Lifespan can be converted to a first-order rate constant.
-
Origination frequency is proportional to BCUs in the mineralization compartment.
-
Resorption rate constant (kSM) is the same whatever the mineralization compartment.
-
Disease progression is due to an increase in bone turnover only.
-
Disease progresses with time and is independent of BMD.
-
Magnitude of the placebo effect is constant over time and does not impact the underlying disease progression.
-
Bisphosphonates inhibit the resorption pathways similarly (same constant whatever the mineralization compartment).
-
Bisphosphonates do not impact the underlying disease progression and placebo effect.
-
BMD at one specific site (e.g. lumbar spine) can be correlated to the overall change in biomarkers such as CTX or osteocalcin.
-
Relationship between CTX, osteocalcin, and BMD under placebo is the same as under bisphosphonates.
Total amount of BCU is based on a distribution of the BCU over the various compartments
Model equations of the various compartments
Baseline or steady state conditions
Lifespan parameters are transferred to first-order rate constants
k SM = 1/lifespan of BCU in slow mineralization process, k RC = 1/lifespan of BCU in resorption cavities, k CF = 1/lifespan of BCU in matrix formation, k FM = 1/lifespan of BCU in fast mineralization phase and k TR = 1/lifespan of BCU in transition in slow mineralization.
Placebo effect
Disease progression
Drug inhibitory function
The biomarker CTX is assumed to be determined by the amount of BCU in the mineralization compartments and the rate of transfer of the BCUs from the mineralization compartment to the resorption compartment.
CTX as change from baseline
where BCU FM and BCU SM represent the amount of BCUs in the fast and slow mineralization compartments, and the parameters BCU FM,0 and BCU SM,0 represents the amounts of BCU at baseline. In this way, the CTX parameters are dimensionless and described as percentage change from baseline.
For the biomarker osteocalcin, the output rate of BCUs from the collagen formation compartment was used, as this biomarker is exclusively released by osteoblasts and is deposited into bone matrix. Since osteocalcin had an apparent residual concentration not related to bone turnover, an empirical correction factor of 1.44 was used.
OC as change from baseline
For BMD, the amounts of BCUs in the fast and slow mineralization compartments were used.
BMD as change from baseline
Rights and permissions
About this article
Cite this article
van Schaick, E., Zheng, J., Ruixo, J.J.P. et al. A semi-mechanistic model of bone mineral density and bone turnover based on a circular model of bone remodeling. J Pharmacokinet Pharmacodyn 42, 315–332 (2015). https://doi.org/10.1007/s10928-015-9423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9423-3