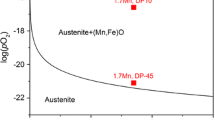
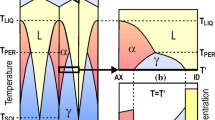
Abstract
In the present work, a 1D implicit model has been developed to simulate simultaneous diffusion of chemical species and precipitation of simple and mixed oxides in the steel matrices for different alloy compositions and dew points. Thermo Gravimetric Analysis has been done by heating the samples (Fe-1.58 wt.% Mn) at 15 K/min and holding them at the peak temperatures of 650 ℃,750 ℃ and 850 ℃ for 2 h in an atmosphere of Ar + CO + CO2, having CO2/CO ratio of 1, 2 and 3 followed by cooling at 15 K/min. From the precipitation simulation at 800 ℃ temperature and −40 ℃ dew point for 1.235, 1.58, 2.52, 3.58 wt. % Mn steel, internal oxidation depth of 0.38 µm was obtained. Increase in dew point results increase of internal oxidation zone’s depth exponentially. The simulation data of Fe-1.225 wt.% Mn-0.075 wt.% Si steel oxidised at 800 ℃ at −40 ℃ dew point shows precipitation of MnO solely at the surface node while SiO2 precipitate over a large region (up to 0.98 µm depth). Parabolic growth followed by linear growth has been observed from the TGA graphs. With increasing temperature, parabolic region decreased and linear growth dominates. Top surface morphology of the oxidised sample changes with increasing temperature and the oxide particle size found to be increase with increasing CO2/CO ratio.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Marder A (2000) The metallurgy of zinc-coated steel. Prog Mater Sci 45:191–271. https://doi.org/10.1016/S0079-6425(98)00006-1
Li F, Liu H, Shi W, Li L (2011) Thermodynamic consideration on selective surface oxidation of high strength steels prior to hot-dip galvanizing. J Coat Technol Res 8:639–647. https://doi.org/10.1007/s11998-011-9334-1
Maki J, Mahieu J, De Cooman B, Claessens S (2003) Galvanisability of silicon free CMnAl TRIP steels. Mater Sci Technol 19:125–131
Gong Y, Kim H, De Cooman B (2008) Formation of surface and subsurface oxides during ferritic, intercritical and austenitic annealing of CMnSi TRIP steel. ISIJ Int 48:1745–1751
Liu H, He Y, Swaminathan S, Rohwerder M, Li L (2011) Effect of dew point on the surface selective oxidation and subsurface microstructure of TRIP-aided steel. Surf Coat Technol 206:1237–1243
Maderthaner M, Jarosik A, Angeli G, Haubner R (2017) Effect of dew point on the selective oxidation of advanced high strength steels. Mater Sci Forum 891:292–297
Olefjord I, Leijon W, Jelvestam U (1980) Selective surface ace oxidation during annealing of steel sheets in H2/N2. Appl Surf Sci 6:241–255
Swaminathan S, Spiegel M (2008) Effect of alloy composition on the selective oxidation of ternary Fe–Si–Cr, Fe–Mn–Cr model alloys. Surf Interface Anal 40:268–272
Wagner C (1959) Zeitschrift fur Elektrochemie 63:772 (in German)
Huin D, Lanteri V, Loison D, Autesserre P, Gaye H (eds) (1997) In microscopy of oxidation—3, newcomb and little. The Institute of Metals, London, pp 573–586
Whittle D, Gesmundo F, Bastow B, Wood G (1981) The formation of solid solution oxides during internal oxidation. Oxid Met 16:159
Rapp R (1965) Kinetics, microstructures and mechanism of internal oxidation—its effect and prevention in high temperature alloy oxidation. Corrosion. 21:382–401
Thermodata (2005) Electronic data bank for thermodynamic quantities, address. http://thermodata.online.fr
Rist A, Ancey-Moret MF, Gatellier C, Riboud PV (1974) Équilibres thermodynamiques dans l'élaboration de la fonte et de l'acier. Techniques de l'ingénieur. Matériaux métalliques 2(M1730):1–42
Swisher JH, Turkdogan ET (1967) Transactions of the metallurgical society of AIME 239:426
Oikawa H (1982) Lattice diffusion in iron-a review. Trans Iron Steel Inst Japan 68:1489
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sadhu, S., Chakraborty, A., Singh, S.B., Halder, A.K. (2022). Investigation of the Selective Oxidation Process for High Strength Steels. In: Kumari, R., Majumdar, J.D., Behera, A. (eds) Recent Advances in Manufacturing Processes. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-3686-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-3686-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3685-1
Online ISBN: 978-981-16-3686-8
eBook Packages: EngineeringEngineering (R0)