Abstract
The industrial solid waste recycling team at North Minzu University has done much research on resource utilization of magnesium slag. This chapter mainly introduces a discussion of resource utilization of magnesium slag. This includes the use of magnesium slag to prepare glass ceramics, porous ceramics, and sulfoaluminate cement clinker, fixing/stabilizing heavy metals in acidic residue generated by lead-zinc smelting, and modifying copper slag.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Preparation of Glass Ceramics Using Mg Slag
5.1.1 Preparation Principle of Glass Ceramics
Glass ceramics are uniform polycrystalline materials in which glass and crystals coexist. They are commonly prepared via the addition of a crystal nucleating agent, forming crystal nuclei in the glass after proper heat treatment followed by crystal nucleus growth; ultimately, glass ceramics are obtained. Compared with other materials, glass ceramics have properties such as an adjustable thermal expansion coefficient (a zero expansion coefficient can be achieved), high mechanical strength, excellent electrical insulation, low dielectric loss, remarkable wear resistance, corrosion resistance, high temperature resistance, and also good chemical stability [1].
Using magnesium slag to prepare glass ceramics can consume and reuse a large amount of industrial waste that is generated by magnesium smelting. Furthermore, the prepared glass ceramics materials have excellent properties. Han Fenglan of North Minzu University prepared glass ceramics via sintering. Magnesium slag, resin ash, and alumina were mixed according to the composition design, and the mixture was melted at high temperature, quenched with water, and ground to fine powder. After the thermal performance was characterized using thermogravimetric analysis and differential scanning calorimetry (TG-DSC), the glass powder was dried, pressed into shape, and then the sample was nucleated (hold time of 1 h) and crystallized (hold times of 0.5, 1, 1.5, 2, and 2.5 h) to prepare glass ceramics [2].
5.1.2 Development of Glass Ceramics in China and the World
Glass ceramics were originally developed from photosensitive glass in 1957. Since Kemantaski in the United Kingdom first used blast-furnace slag to prepare glass ceramics in 1965 [3], glass ceramics were rapidly developed from specific application to extensive uses in architecture and other fields [4]. With the development of the technological economy, glass ceramics have been successfully commercialized. In Europe and America, rock glass ceramics and slag glass ceramics were first industrialized for use in decoration materials in buildings. In the mid-1960s, the former Soviet Union reported that slag glass ceramics could be used as building materials in practical production. In the early 1970s, Czechoslovakia used molten-cast basalt to manufacture wear-resistant flooring materials. At the same time, the production of rock-ceramic glass for use as decorative panels in construction also emerged in United States [5, 6]. In Western countries, lithium-based glass ceramic materials have been used to manufacture optical fiber connectors. The thermal expansion coefficient and hardness of lithium-based glass ceramics match those of optical fiber connecters made of silica glass better than traditional zirconia. In addition, lithium-based glass ceramics are more easily machined with high precision and have excellent environmental stability.
In Asia, Japan was the first to develop and put glass ceramics into practical use for buildings; the melt-sintering method was mainly used to produce artificial marbles with a glass ceramic structure. South Korea also produces high-grade glass ceramic decorative panels. In China, glass ceramics are well-developed and are used in building materials and mechanical engineering because, in addition to other advantages, they have good corrosion resistances, wear resistances, good insulation, light specific gravity, and can be seamlessly welded with metals. The steel slag-based glass ceramics that have been developed via sintering formation in China are compact and have a smooth surface and low porosity; thus, they have promising market values. Slag glass ceramics “consume waste slag” and also have the advantages of excellent performance, a simple preparative technique, and practical scalability production; hence, it has always attracted the attentions of material scientists and engineers in various countries. In recent years, the use of slags to prepare glass ceramics as a reasonable method to recycle slag has attracted increasing attention [6,7,8,9,10].
5.1.3 Preparation of Glass Ceramics Using Slags
The preparation process of glass ceramics generally includes melting, sintering, and the sol-gel method [11, 12]. Among these, melting and sintering are the main preparation methods.
Melting method
Melting is the first method proposed for preparing glass ceramics. The main process is to mix and grind the nucleating agent with other raw materials first, and then the mixture is melted in a high-temperature environment with a temperature range from 1300 to 1600 °C. After the mixture is completely melted, homogenized, and molded, it is annealed and then nucleated and crystallized using a certain heat treatment process to obtain a uniform and dense glass ceramic sample.
Sintering method
The sintering method was first proposed by Xuanboen around 1960 to produce glass ceramics; this method was used to successfully implement industrial production in Japan around 1970. The process flow is as follows: raw materials are mixed, melted, water quenched, smashed, sieved, shaped, sintered, and deep processed, and thus, products are obtained.
The sintering method overcomes the drawbacks of the melting method, which involves inseparable melting and molding, difficulties in controlling molding process at high temperature, and the need for a nucleating agent. Thus, the prepared glass ceramics have better plasticity. Therefore, the ceramics are more suitable for molding at low temperatures and favorable for industrial production. In addition, the water-quenched glass ceramics exhibit a better overall crystallization phenomenon [13, 14].
5.1.4 Experimental Preparation of Glass Ceramics Using Mg Slag
In this experiment, magnesium slag, resin ash, and alumina were used to prepare glass ceramics via sintering. Magnesium slag was provided by Ningxia Huiye Magnesium Co., Ltd., resin ash powder was provided by a foundry in Ningxia, and alumina powder was provided by Tianjin Guangfu Fine Chemical Research Institute. The compositions of the raw materials are shown in Table 5.1. According to the compositions of the raw materials, the glass ceramic system selected for the experiment is the CMAS (CaO-MgO-Al2O3–SiO2) system; the contents of magnesium slag, resin ash, and alumina are 48.78, 48.78, and 2.44%, respectively [2].
The raw materials were weighed in proportion, placed in a mortar, and manually ground for about 20 min to prepare the mixture. The prepared mixture was placed in a corundum crucible suspended in the middle of a vertical tube furnace; the temperature was increased to 800 °C at a heating rate of 8 °C/min, and the temperature was maintained at 800 °C for 2 min. Next, the temperature was increased to 1385 °C at a heating rate of 5 °C/min, and then, that temperature was held for 90 min.
At the end of the temperature holding time, the corundum crucible and the molten glass were quenched in a water tank located below the vertical tube furnace. This allows the high-temperature molten glass to be instantly cooled. After cooling, the glass sample and the corundum crucible were placed in a drying oven at 100 °C for 20 min. The prepared glass was removed from the corundum crucible and ground in a vibration mill for 30 s to obtain glass powder that had a size of about 400 mesh (37 μm).
To determine the parameters of following sintering process, the obtained glass powder was analyzed using TG-DSC. In sintering process, the powder was pressed into discs that had a diameter of 25 mm under 100 MPa in a dry press for 40 s. The discs were then placed in a vertical tube furnace for sintering. The temperature was increased from room temperature to 500 °C at a heating rate of 10 °C/min. After this temperature was held for 5 min, the temperature was increased to 845 °C at a heating rate of 5 °C/min; this temperature was held for 60 min, and then it was increased to 1021 °C at a heating rate of 3 °C/min. After 150 min of temperature holding, the samples were naturally cooled, and glass ceramics that were made from magnesium slag were obtained.
5.1.5 Property, Morphology, and Phases of Mg slag based Glass Ceramics
The TG-DSC diagram of the prepared glass powder is shown in Fig. 5.1, and the detection instrument was a German Netzsch STA449 thermal analyzer. Glass ceramics need to absorb relatively high energy during nucleation, and so, the temperature 775 °C that corresponds to the maximum endothermic peak is determined to be the nucleation temperature of the glass ceramics. The internal state of glass ceramics tends to be stable during crystallization; the internal energy is subsequently released to form crystals. Therefore, the energy of the glass ceramics is lowest when the ceramics are in the crystalline state. As seen, the trough corresponding temperature in the DSC spectrum is 1021 °C, and this is the crystallization temperature.
Volume density ρ (g/cm3) can be calculated according to following equation:
where:m0 denotes the mass of dry sample in air (g); m1 denotes the mass of water-saturated sample in air (g); m2 denotes the mass of water-saturated sample in water (g); w denotes the density of distilled water at room temperature (g/cm3)
Water adsorption rate Wa (%) is calculated according to the following equation:
The changes in the volume density of the glass ceramic sample with respect to the holding time are shown in Fig. 5.2. As seen in Fig. 5.2, when the holding time was longer, the volume density gradually increased. In the interval from 0.5 to 1 h, the volume density of the sample basically remained constant. From 1 h to 2 h, the volume density of the sample increased rapidly until it reached a maximum value of 2.77 g/cm3 when the holding time was 2 h. Conversely, the volume density of the sample decreased slightly util the holding time reached 2.5 h.
In the period from 0.5 to 1.5 h, the water adsorption rate of the sample first increased and then decreased. After 1.5 h, the decreasing trend slowed down, and the water adsorption rate was almost parallel to the X axis. The data shows that with holding time longer than 1.5 h, the internal structure of the glass ceramic sample became denser, and the water absorption rate was very low. The water absorption rate reached a maximum value of 5.13% when the holding time was 1 h. When the holding time was 2.5 h, the water adsorption rate of the sample reached the lowest value of only 0.07%.
The samples with holding times of 1, 1.5, 2, and 2.5 h were scanned using a scanning electron microscope (Zeiss EVO-18, Germany), and the microstructures were observed. SEM images of the glass ceramics with different crystallization holding times are shown in Fig. 5.3. In the figure, panels (a), (b), (c), and (d) show the sample microstructures when the holding times were 1, 1.5, 2, and 2.5 h, respectively.
It is clear from Fig. 5.3 that with longer crystallization holding time, the glass ceramics made from magnesium slag became increasingly denser, and the porosity decreased. It can be seen that the extended crystallization holding time enhanced the density of glass ceramics.
In Fig. 5.4, the upper left pattern corresponds to the sample that had a holding time of 0.5 h; the upper right pattern corresponds to the sample that had a holding time of 1.5 h; the bottom pattern corresponds to the sample with a holding time of 2.5 h. It can be seen that the main crystal phase in Mg slag glass ceramics is diopside (CaMg (SiO3)2) with a small amount of inclined top flint (MgSiO3).
5.2 Preparation of Porous Ceramics Using Mg Slag
Porous ceramics are a new type of ceramic material. Porous ceramics are sintered at a high temperature and have a large number of interconnected or closed pores that form in the material body during the formation and sintering process. The advantages of porous ceramic materials are that they are environmentally friendly and have high porosity, low air resistance, stable chemical properties, good regeneration performance, and good resistances to high temperature, high pressure, and chemical corrosion. Thus, porous ceramic materials have been rapidly developed in recent years and have been widely used in filtration, purification and separation, catalyst carriers, sound and shock absorption, thermal insulation materials, biological materials, sensors, and aeronautical and aerospace materials [15,16,17].
5.2.1 Characteristics of Porous Ceramics
-
(1)
High porosity. Porous ceramics are characterized by a large number of uniform pores with controllable sizes. The pores are classified as open pores and closed pores. The functions of open pores include filtering, absorbing, adsorbing, and eliminating echoes, while closed pores are favorable for insulating heat and sound, and transfer of liquid and solid particles.
-
(2)
High strength. Porous ceramics are generally prepared from metal oxides, such as silicon dioxide and silicon carbide, and are sintered at high temperatures. All of these materials have intrinsic high strength. During the calcination process, the boundary regions of these raw material particles melt and bond, thereby forming ceramics that have higher strength.
-
(3)
Stable physical and chemical properties. Similar to other ceramic materials, the advantages of porous ceramics are that they have good acidic and alkaline corrosion resistance and withstand high temperature and high pressure. In addition, porous ceramics have a good self-cleaning ability and do not cause secondary pollution. Thus, they are green and environmentally friendly functional materials.
-
(4)
High filtration accuracy and good regeneration performance. Porous ceramic materials that are used as filter materials have narrower pore size distribution, higher porosity, and higher specific surface area. The porous ceramics fully contact the filtered materials, and pollutants such as suspended solids, colloids, and microorganisms are blocked on the surface or inside of the filter medium, exhibiting a better filtering effect. After a period of use, the porous ceramic filter material can be backwashed with gas or liquid to restore its original filter capacity.
5.2.2 Preparation of Porous Ceramics Using Mg Slag
5.2.2.1 Selection of Raw Materials
Porous ceramics that are commercially available mostly use materials such as Al2O3, SiC, and mullite, as the main raw materials. These materials have relatively high prices and complicated preparation processes, and these characteristics limit the promotion and applications of porous ceramics [16, 17]. The main components of magnesium slag, fly ash, and acetylene sludge are SiO2, CaO, and Al2O3, which are similar to the components of commercially available porous ceramics materials; thus, solid wastes like magnesium slag can be used as raw materials to prepare porous ceramics [18,19,20]. Magnesium slag is mainly composed of CaO, SiO2, and Al2O3, and fly ash is mainly composed of CaO, SiO2, Al2O3, and MgO. Fly ash is the main solid waste discharged from coal-fired power plants and one of the biggest displacement industrial wastes in China. Acetylene sludge is solid waste obtained after acetylene is prepared from calcium carbide, and it is composed mainly of CaO and Ca(OH)2. It also contains SiO2 and Al2O3 and a small amount of CaCO3, Fe2O3, MgO, carbon slag, and other components. Wanxiu et al. [21] used three industrial solid wastes (magnesium slag, fly ash, and acetylene sludge) as raw materials; the raw materials were mixed in a certain ratio, pressed, and sintered to obtain porous ceramics containing silicate phases.
5.2.2.2 Preparation Process of Porous Ceramics Using Magnesium Slag
The industrial solid waste magnesium slag used in the experiment was provided by Ningxia Huiye Magnesium Co., Ltd. The fly ash was provided by Ningxia Shenhua methanol plant. The acetylene sludge was from Ningxia Dadi chemical company. The chemical compositions of magnesium slag, fly ash, and acetylene sludge are shown in Table 5.2.
5.2.2.3 Properties of Porous Ceramics Made from Magnesium Slag
The raw materials were weighed according to different formulations then ground and mixed in a vibrating mill with a mixing time of 60 s. The mixture was unidirectionally pressed under a pressure of 63.7–95.9 MPa, and the holding time was 50-60 s. Pressureless sintering were carried out at a sintering temperature of 1150 °C, a heating rate of 10 °C/min, and a holding time of 4 h. After the sintering was complete, the samples were furnace-cooled to room temperature; then, the samples were removed, and their physical and chemical properties were measured.
5.2.2.4 Properties of Magnesium Slag Porous Ceramics
The experimental results of the sample that was sintered for 4 h at 1150 °C show that the prepared porous ceramic has a maximum compressive strength of 98 MPa when the ratio of magnesium slag to fly ash to acetylene sludge was 70:25:1. The porous ceramic has the largest porosity of 57% when the ratio of magnesium slag to fly ash to acetylene sludge was 12:3:5. When the magnesium slag:fly ash:acetylene sludge ratio was 6:3:1, the porous ceramics have a complete framework and uniformly distributed micropores. Adding acetylene sludge and carbon powder as pore-forming agents can homogenize the pore distribution, refine pore size, enhance the porosity, and gas filtration performance of porous ceramics. With the same amount of pore-forming agent, the carbon powder has a better pore-forming effect; specifically, the weight loss, water absorption, and porosity of the porous ceramics reached maximum values of 30, 38, and 53%, respectively, and the minimum volume density was 1.4 g/cm3. The main phases in porous ceramics are mainly calcium metasilicate, dicalcium silicate, or calcium magnesium silicate, which are the products of the high-temperature reaction between CaO and SiO2, and small amount of aluminosilicate and ferrite.
The typical microstructure of porous ceramics that are made from magnesium slag is shown in Fig. 5.5. For the sample in the photo, the ratio of magnesium slag:fly ash:acetylene sludge was 80:5:15. The weight loss of the sample was 10.33%; the porosity was 38.86%; the water absorption was 21.52%; the bulk density was 1.78 g/cm3; the compressive strength was 30.10 MPa.
5.3 Preparation of Slag-Sulphoaluminate Cement Clinker Using Mg Slag
Magnesium slag has a metastable high-temperature structure, and it has active cations and a high hydration activity. Liguang et al. [22] mixed magnesium slag, mineral slag, and cement clinker to prepare magnesium slag-based cementitious materials. The effects that the magnesium slag content, cement clinker content, grinding process, and auxiliary activator have on the strengths (compressive and flexural strength) of cementitious materials that are made from magnesium slag were discussed. The hydration products of magnesium slag cementitious materials were analyzed. Fenglan et al. [23] used magnesium slag to prepare cementitious materials. From this research, they summarized the following: magnesium slag has hydration activity and can form calcium silicate cement after hydration, and its strong hygroscopicity contribute to the durability and strength of cement mortar. Because of these characteristics, different contents of ground magnesium slag power were added to cement (42.5) clinker. After studying its water consumption, setting time, flexural strength, compressive strength, and stability, it was concluded that it is feasible to add 15-20% of magnesium slag without affecting the cement performance indexes.
Sulphoaluminate cement has become one of the most active research hotspots because it has the characteristics of a low sintering temperature, low emission, high early-stage strength, good impermeability, and resistances to frost and corrosion. Ordinary sulphoaluminate cement is mainly prepared from limestone, alumina, and gypsum as raw materials. In recent years, to increase the resource utilization rate of industrial solid waste, researchers have conducted a lot of research in the preparation of belite sulfoaluminate cement using industrial solid wastes such as fly ash, coal gangue, and phosphogypsum as raw materials [24,25,26,27]. Zhao et al. [28] mixed electrolytic manganese residue and magnesium slag to sinter sulphoaluminate cement clinker, and then, they carried out a series of experimental studies, which are described in detail below.
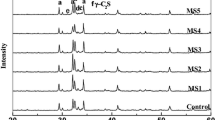
5.3.1 Raw Materials of Slag-Sulphoaluminate Cement
Experimental materials: Electrolytic manganese residue was provided by Ningxia Tian Yuan Manganese Co., Ltd., and magnesium slag was provided by Ningxia Huiye Magnesium Co., Ltd. CaSO4·2H2O was from Tianjin Komiou Chemical Reagent Co., Ltd.; Fe2O3, SiO2, Al2O3, and CaO are commercial reagents that were all analytically pure. As seen in Fig. 5.6, the phases in the electrolytic manganese residue (Fig. 5.6 a) are mainly CaSO4·2H2O and SiO2. The phases in magnesium slag (Fig. 5.6 b) are mainly C2S, MgO·Fe2O3, CaO·MgO·SiO2, of which C2S is the primary part. Dihydrate gypsum and C2S are the main components of sulphoaluminate cement. After calcination, dihydrate gypsum decomposes to form SO3, and this is beneficial for the formation of calcium sulfoaluminate; C2S can enhance the later strength of sulfoaluminate cement.
Sulfoaluminate cement clinker is composed of minerals such as anhydrous calcium sulfoaluminate (C4A3Š), dicalcium silicate (C2S), and calcium aluminate ferrite (C4AF). (In these abbreviations, the letters have the following designations: C-CaO, A-Al2O3, Š-SO3, S-SiO2, and F-Fe2O3). According to the mineral composition of sulfoaluminate cement and process control conditions, the designed composition of the experimental sample is shown in Table 5.3. The sintering temperatures are 1200, 1230, 1260, and 1300 °C, and the temperature was held for 30 min. To completely sinter the cement clinker in the muffle furnace, the sintering process was repeated twice.
5.3.2 Phases in Cement Clinker
X-ray diffraction was used to analyze the phase composition of the sintered sulphoaluminate cement clinker. Figure 5.7 shows the phases in the sulphoaluminate cement clinker sample that was sintered at different temperatures. The main mineral phases in the clinker samples that were sintered at 1200, 1230, and 1260 °C are C2S and C4A3Š, and basically, there are no other phases. As seen from these three patterns, the intensities of the diffraction peaks of C2S and C4A3Š in the sample that was sintered at 1260 °C are much greater than those in the samples that were sintered at 1200 and 1230 °C. For the sample that was sintered at 1300 °C, the useless C2AS with no hydration activity appears in the cement clinker, and its diffraction intensity is much higher than those of the useful components, such as C2S and C4A3Š. C4AF is basically not observed in the figure because the iron phases in manganese slag and magnesium slag are equivalent to that in solid solutions (with lower melting point) during the calcination of cement clinker. Part of the solid solution enters into C4A3Š, and this results in lower Fe content in C4AF. Some of the intermediate product that forms C4AF reacts with anhydrite to form C4A3Š, and this leads to higher C4A3Š content in the clinker and a decrease in the C4AF content [29, 30]. Therefore, through comprehensive analysis, it can be determined that 1260 °C is the optimal sintering temperature. Compared to the sintering temperature (1450 °C) of traditional Portland cement clinker, the sintering temperature in this experiment was much lower. The reason for this is that Fe oxides as solid solutions greatly reduce the sintering temperature of raw materials, and thus, slag that contains a small amount of iron phase reduces the sintering temperature of raw materials. Therefore, when manganese slag and magnesium slag are used to prepare sulfoaluminate cement clinker, the energy consumption can be reduced, the heating process can be easily controlled, and the sintering cost can be reduced.
5.3.3 Experimental Results
When different amounts of gypsum were added to the prepared cement clinker, a TAM Air eight-channel isothermal microcalorimeter (Retrac HB, Sweden) was used to determine the heat of hydration, to determine the optimal gypsum dosage, and to analyze the effect of different hydration times on the hydration performance. Compressive and flexural properties of the prepared sulphoaluminate cement clinker were tested according to the Method of testing cements-Determination of strengths (GB/T 17671-1999). The impermeability of the material was measured with reference to the impermeability test specification of cement mortar. According to the toxic leaching method in HJ/T 299-2007 “Solid waste-Extraction process for leaching toxicity-Sulfuric acid and nitric acid method”, sulfuric acid and nitric acid were used as the leaching agents to perform the toxicity leaching of different samples, and residues was determined. The concentration of heavy metal ions in the leaching solution was compared to the limit in the Integrated Wastewater Discharge Standard (GB 8978-1996).
The experimental results show the following: (1) The content of electrolytic manganese residue and magnesium slag in the raw meal can each reach 21%. The optimal sintering temperature was 1260 °C, and the holding time was 30 min. At this point, the mineral phase in the sintered sample was mainly C2S and C4A3Š. (2) A certain amount of gypsum was added to the prepared cement clinker. When the added amount was 15%, the hydration heat released reached a maximum, and the mechanical properties were the best. The flexural strength at 28 d was 5.1 MPa, and the compressive strength was 31.2 MPa. The impermeability level reached P6, and the sintered clinker and hydration products effectively solidified and stabilized the heavy metals in the industrial solid waste; this made it difficult for them to be leached. The following conclusions can be drawn: through sintering, the use of electrolytic manganese residue and magnesium slag can produce qualified fast-hardening sulphoaluminate cement clinker with higher early-stage strength. Compared to commercial silicate and sulphoaluminate cement clinkers, the cement clinker prepared using magnesium slag has advantageous properties, is low cost, can turning waste into treasure, and creates a low amount of pollution.
5.4 Solidification/Stabilization of Heavy Metals in Industrial Solid Waste
5.4.1 Fixation and Stabilization of Heavy Metals in Pb/Zn Smelting Slags
Solidification is a process in which appropriate additives are used in toxic and hazardous wastes to stably fix the toxic and hazardous substances in the waste. This eliminates pollution and damage caused by the waste to the surrounding ecosystem through reducing the leaching or release of toxic and hazardous components. Toxic and harmful pollutants are decomposed, precipitated, neutralized, or transformed into low-migration, low-dissolution, low-toxic, or even non-toxic matters through treatments; thus, environmental pollution caused by toxic and hazardous substances can be reduced. Because most of the materials used to treat toxic and hazardous solid wastes have both stabilizing and fixing effects, the decontamination and detoxication for waste is usually called stabilization/solidification technology, and this is sometimes shortened to solidification [31]. At present, stabilization/solidification technology has been widely used in the treatments for contaminated sites and solid waste. Compared with other technologies, such as chemical treatment or bio-remediation, stabilization/solidification technology has the advantages of convenient construction and low cost [32]. The curing agents that are used in stabilization/solidification technology include cement, slag, fly ash, quicklime, pharmaceuticals, organic polymers, geopolymers, modified clays, and certain waste materials [33]. The geopolymer is polymerized by inorganic silicon-oxygen tetrahedra and aluminum-oxygen tetrahedra. When the geopolymers are used to fix wastes, the process is simple and is more stable.
Chen et al. used magnesium slag and fly ash-based geopolymers to solidify/stabilize acidic residue generated via Pb-Zn smelting and conducted a large number of experimental studies [32, 34, 35]. These are described in detail below.
Lead and zinc smelting is an important case of China’s nonferrous metal smelting. The bulk of industrial solid waste from lead and zinc industries in China mainly come from metallurgical slag and the residue that is generated via acidic water treatment (acidic residue). The amount of annual industrial waste that is generated from lead/zinc production is estimated to exceed 6 million tonnes. When the waste slag is piled up, the heavy metal in the slag migrates and causes serious pollution in the surrounding water. At present, heavy metal pollution in China has become an increasingly serious and prominent environmental problem that causes serious damage to the health of citizens [36]. Magnesium slag and fly ash-based geopolymers are used to solidify/stabilize heavy metals in acidic residue, and this can prevent the heavy metal pollutants in waste from entering the environment again and causing secondary pollution. Ultimately, this achieves the purpose of treating waste using waste and the efficient utilization and savings of resources.
5.4.2 Morphological Analysis of Heavy Metals Pb and Cd in Acidic Residue
The BCR three-step extraction method was used to perform leaching and extraction experiments on acidic residue samples. It was found that the morphology distribution of Pb in the acidic residue was in the order of oxidizable state > reducible state > residue state > extractable state by acid. Among these states, the oxidizable state accounts for 69.02% of the total Pb. The state distribution of Cd in the acidic residue is in the order of extractable state by acid > oxidizable state > reducible state > residue state; among these, the content of the extractable state by acid accounts for 80.89% of the total Cd. The doping of magnesium slag promotes the conversion of the heavy metals Pb, Cd, Cu, and Zn in the waste slag from an unsteady state to a stable state, and this achieves the purpose of solidifying/stabilizing the heavy metals Pb, Cd, Cu, and Zn [34].
5.4.3 Experiments and Research Method
Main research methods: (1) Chemical analysis methods and X-ray fluorescence spectrometry (XRF) are used to determine the main chemical compositions and the percent of each compound in industrial solid wastes such as magnesium slag, fly ash, and acidic residue; X-ray diffraction (XRD) is used to determine the main phases of the solid wastes. (2) The morphology distribution of Pb, Cu, and Cd in acidic residue is analyzed using the BCR long-range order extraction method; magnesium slag is used to solidify/stabilize heavy metals in acidic residue, and the morphology distribution of heavy metals in the solidified/stabilized waste residue is analyzed. Toxicity leaching experiment are performed on the samples, and inductively coupled plasma emission spectrometry (ICP-7000) is used to determine the contents of heavy metals in the leaching solution. XRD, scanning electron microscopy with Energy dispersive X-ray spectroscopy (SEM/EDX) and Fourier transform infrared spectroscopy (FTIR) are used to study the phase composition and microstructure of the waste residue before and after the toxicity leaching experiment. (3) Fly ash and alkali activator are used to prepare fly ash-based geopolymer. The prepared geopolymer that has good mechanical properties is used to solidify/stabilize the heavy metals Pb and Cd. The mechanical properties of the fly ash-based geopolymer are determined, and a toxicity leaching test is carried out to determine the amounts of Pb and Cd in the leached solution.
The experimental research is divided into four parts: (1) solidification/stabilization of heavy metals experiment using magnesium slag and acidic residue at high temperature; (2) solidification/stabilization of heavy metal experiment using magnesium slag and acidic residue at room temperature; (3) preparation of fly ash-based geopolymers; (4) solidification/stabilization of heavy metals using the fly ash-based geopolymers. The process routes are shown in Figs. 5.8, 5.9, 5.10, 5.11.
The contents of heavy metal elements in magnesium slag and acidic residue are shown in Table 5.4. As seen in Table 5.4, the amounts of harmful heavy metal elements in magnesium slag are relatively low. Thus, the environmental hazards caused by heavy metals in magnesium slag can be ignored. Also, the amounts of the heavy metals Pb, Zn, and Cd in acidic residue are relatively high.
Pb(NO3)2 and Cd(NO3)2·4H2O are dissolved in distilled water to prepare solutions with different concentrations of Pb and Cd. The solutions are added to the original acidic residue; then, the mixture is stirred, dried, and ground to obtain acidic residue samples with different concentrations of Pb and Cd. The sulfuric acid and nitric acid method (HJ/T299-2007) is used to perform a toxicity leaching experiment. For the leaching solutions obtained in the toxicity leaching experiments, the Pb concentrations in all of the samples (including the original acidic residue without added Pb), exceed the concentration limit of Pb ≤ 5 mg/L in GB5085.3-2007. The Cd concentrations of all of the samples with added Cd exceed the limit of Cd ≤ 1 mg/L in GB5085.3-2007. It is observed that the amounts of the heavy metals Pb and Cd in the acidic residue discharged from lead-zinc plants are relatively high. Although the enriched amount does not have a recycling value, the leaching toxicity far exceeds the national standard, and thus, it needs to be solidified/stabilized before it is discharged.
5.4.4 Solidification/Stabilization of Heavy Metals Using Mg Slag and Acidic Residue at High Temperature
To stabilize the heavy metals in the acidic residue and to reduce their environmental pollution, magnesium slag was mixed with acidic residue to achieve the fixation/stabilization of heavy metals at high temperature according to the characteristics of magnesium slag.
Acidic residue (acidic residue + heavy metal) samples with heavy metal content exceeding the limits in the standard were prepared. The samples were mixed with magnesium slag at a ratio of 40:60%. The mixture was pressed into blocks using a hydraulic press, and they were sintered in a box-type resistance furnace at a temperature of 1200 °C. The temperature was maintained for 6 h; then, after the samples were cooled in the furnace, they were removed, and the toxicity leaching experiment was carried out. The experimental results indicate that when the amounts of Cd, Cu, and Pb reach 1.070, 2.471, and 0.38%, respectively, in the samples, the concentrations of Cd, Cu, and Pb in the leaching solution meets the critical limit in the GB5085.3-2007 standard. This shows that the Cd, Cu, and Pb in the samples can exist stably and are not easily leached after the samples were doped with 60% magnesium slag and sintered at 1200 °C for 6 h.
XRD phase analysis shows that after sintering, a part of γ-C2S phase in the sample transformed into the β-C2S phase, and this increased the amount of β-C2S and decreased the amount of γ-C2S. This is because γ-C2S is the stable phase of Ca2SiO4 at normal temperature, and β-C2S is the high-temperature stable phase of Ca2SiO4. After high temperature treatment, the γ-C2S in the sample converted into β-C2S. A higher amount of β-C2S crystalline phases is conducive to the solidification/stabilization of heavy metals, such that heavy metals are stabilized in slag and are not easily leached. Compared with the nonsintered sample with the same composition, the leaching solution of the sintered samples had lower observed concentrations of heavy metals in leaching solution.
5.4.5 Solidification/Stabilization of Heavy Metals Using Mg Slag and Acidic Residue at Room Temperature
A certain quality of heavy metal salts containing Pb, Cu, and Cd was dissolved in distilled water to obtain heavy metal solutions with different concentrations. The solutions were poured into a mixture of magnesium slag and acidic residue to ensure that heavy metals were present as free ions in the slags. After standing for 5 min, the samples were dried, crushed, and ground. The treated samples were subjected to toxicity leaching experiment using the sulfuric acid and nitric acid method (HJ/T299-2007).
The main component of magnesium slag is C2S, and its structure is a metastable high-temperature structure. Magnesium slag has a high hydration activity and can form calcium silicate hydrate gel (C-S-H gel) after hydration. Consequently, the heavy metals in the slag can be effectively solidified/stabilized in the slag and cannot be easily leached when the acidic residue is doped with magnesium slag.
The Pb in the original acidic residue was unstable and is easily leached. For the original acidic residue without doped with magnesium slag, when the Pb content in the original acidic residue reached 1.810%, the Pb concentration of the leaching solution exceeded the limit determined by GB5085.3-2007. For the same residue sample but was doped with 80% magnesium slag, the Pb concentration met the standard. Even, when the original Pb content increased to 2.650% in the sample doped with 80% magnesium slag, the Pb concentration of the leaching solution still met the standard. These results indicated that doping with 80% magnesium slag, Pb in the slag material is stable and not easily leached.
When the acidic residue is mixed with 10 and 20% magnesium slag, both Cd and Cu are stable in the slag and are not easily leached. The leaching rate is defined as the ratio between the heavy metal content of the leaching solution of treated samples and that of the original slag. When the leaching rate is smaller, the fixing/stabilizing effect is better. Experimental results indicate that when the doping level of magnesium slag is 10 and 20%, all of the Pb, Cu, and Cd concentrations of the leaching solution of acidic residue meet the GB5085.3-2007 standard.
References
Liu Y (2006) Preparation and study on slag glass-ceramics. Dissertation, Hunan University
Su L (2018) Study on properties of glass-ceramic made from magnesium reduction slag. North Minzu University, Thesis
Nan X (2006) Preparation of glass-ceramic. Dissertation, Lanzhou University of technology
Yoon S, Lee J et al (2013) Characterization of wollastonite glass-ceramics made from waste glass and coal fly ash. J Mater Sci Technol 29(2):149–153
Chen F, Zhao E et al (2007) The research development and application of CaO-Al2O3-SiO2 glass for decoration. Ceramics 2(20):3–4
You X (2014) Review on the preparation of glass-ceramics from fly ash. Bullet Chinese Ceramic Soc 33(11):2902–2907
Chen G, Liu X et al (2002) Slag glass-ceramics: fabrications and propects. ceramics 4(16):2–3
Chen H (1988) Study on slag based glass ceramics. Glass and Enamel 16(2):1–7
Li J, Qian W et al (1992) Machinable glass-ceramics made from industrial slags. J Inorgan Mater 7(2):3–4
Chen G (1993) Microcrystallization of CaO-Al2O3-SiO2 Syst. Glass 6:1–6
Zhao B (2010) Experimental study on glass-ceramic preparation from sewage sludge ash by microwave melting method. Dissertation, Harbin Institute of Technology
Ma X (2011) Classification and application of polarization glass. World of Building Mater 32(3):12–15
Yao Q (2005) The process and property investigation of steel slag glass-ceramics. Dissertation, Nanjing university of technology
Xiao HN, Deng C et al (2001) Effect of processing conditions on the microstructure of glass-ceramics prepared from iron and steel slag. J Human Univer Natural Sci 28(1):32–35
Zeng L, Hu D et al (2008) The novel techniques and development of preparation of porous ceramics. China Ceramics 44(7):7–11
Huang X, Ma X et al (2015) Current situation of preparation and application of porous ceramic materials. China Ceramics 9:5–8
Ju Y, Song S et al (2007) The preparation, applications and research development of the porous ceramics. Bullet Chinese Ceramic Soc 26(5):969–976
Li X, Zhang S et al (2011) Review on the recycle of magnesium slag wastes. Concrete 8:97–101
Lei R, Fu D et al (2013) Research progress of fly ash comprehensive utilization. Clean Coal Technol 3:106–109
Wang H, Tong J et al (2007) Resourcification utilization routes for carbide slag. Chem Prod Technol 1:47–53
Hai W, Han F et al (2018) Influence of ratio of raw materials on the properties and morphology of industry solid wastes porous ceramics. Bullet China Ceramic Soc 37(12):3776–3780
Xiao L, Luo F et al (2009) The analysis on mechanism of using magnesium slag to prepare the cementitious material. J Jilin Jianzhu Univer 26(5):1–5
Han F, Zhou S et al (2013). Cementing material preparation from magnesium slag. In: Proceeding of annual conference of chinese society for environmental sciences, pp 5554–5557
Zhang H, Li H et al (2014) Preparation of Belite-sulphoaluminate cement using phosphogypsum. Bullet China Ceramic Soc 33(6):1567–1571
Arjunan P, Michael R et al (1999) Sulfoalu minate-belite cement from low-calcium fly ash and sulfur-rich and other industri-al by-products. Cement Concrete Res 29:1305–1311
Wan X, Zhang M et al (2010) Study on treatment of metallurgical solid wastes by sulphoaluminate cement. Environ Eng 01:73–76
Xu G (2009) Research of utilizing coal gangue in Shi zuishan district to produce series of sulphoaluminate cements. Dissertation, Chengdu University of Technology
Zhao S, Han F et al (2017) Preperation of composite slag sulphoaluminate cement clinker from electrolytic manganese-magnesium. Bullet China Ceramic Soc 36(05):1766–1772 + 1776
Yuan Y, Ye Z et al (2012) Influence of iron phase composition on barium calcium sulphoaluminate cement. J Univer Jinan (science and technology) 26(2):128–131
Feng X, Zhu Y et al (1984) Investigation on strength development of C4AF and a new type of cement with high early strength and high iron content. J Chinese Ceramic Soc 12(1):32–47
Zhou S (2015). Solidification/stabilization of heavy metals in industrial solid waste. Dissertation, Jiangsu university of technology
Chen Y, Han F et al (2015) Solidification/stabilization of heavy mental Cu and Cd in waste acid residue by magnesium slag. Inorgan Chem Indus 47(7):48–51
Du Y, Jin F et al (2011) Review of stabilization/solidification technique for remediation of heavy metals contaminated lands. Rock Soil Mech 32(1):116–124
Chen Y (2016) Research on the solidification and stabilization of heavy mental by magnesium slag and geopolymer based on fly-ash. Dissertation, North Minzu University
Chen Y, Han F et al (2016) Solidification and stabilization of heavy mental Pb in waste acid residue by magnesium slag. Chinese J Environ Eng 10(06):3229–3234
Zhang J, Wei J et al (2014) Legislation outline on heavy metal pollution prevention. Environ Sustain Develop 1:60–62
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Wu, L., Han, F., Liu, G. (2021). Resource Utilization of Magnesium Slag. In: Comprehensive Utilization of Magnesium Slag by Pidgeon Process. SpringerBriefs in Materials. Springer, Singapore. https://doi.org/10.1007/978-981-16-2171-0_5
Download citation
DOI: https://doi.org/10.1007/978-981-16-2171-0_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2173-4
Online ISBN: 978-981-16-2171-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)