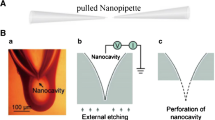
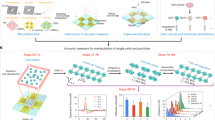
Abstract
Single-cell analysis is an essential tool with numerous applications in biological and medical analyses. Nanophotonic techniques are emerging methods that use nanoscale devices and electromagnetic waves to extract information from a single cell. These techniques generally use the visible spectrum or the near-IR region of the electromagnetic spectrum. Various techniques have been developed by researchers, each for a specific purpose. These methods include mass spectrometry, quantum dots, nanolasers and spasers, optofluidics, zero-mode waveguides, nanoantennas, etc. This chapter gives an overview of these techniques when applied for single-cell analysis. Their basic working principles, key variants, and benefits are explained in detail.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wang D, Zhao X, Gu Z. Advanced optoelectronic nanodevices and nanomaterials for sensing inside single living cell. Opt Commun. 2017;395:3–15. https://doi.org/10.1016/j.optcom.2016.03.047.
Lumen unique characteristics of eukaryotic cells - lumen microbiology.
Schwanhüusser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–42. https://doi.org/10.1038/nature10098.
Szabo DT. Transcriptomic biomarkers in safety and risk assessment of chemicals. In: Biomarkers in toxicology. New York, NY: Academic Press; 2014. p. 1033–8.
Wang D, Bodovitz S. Single cell analysis: the new frontier in “omics”. Trends Biotechnol. 2010;28:281.
Daviss B. Growing pains for metabolomics. Scientist. 2005;19:25–8.
Zenobi R. Single-cell metabolomics: analytical and biological perspectives. Science. 2013;342:1243259. https://doi.org/10.1126/science.1243259.
Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis. 1998;19:1853–61. https://doi.org/10.1002/elps.1150191103.
Blackstock WP, Weir MP. Proteomics: quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999;17:121–7.
Wu AR, Wang J, Streets AM, Huang Y. Single-cell transcriptional analysis. Annu Rev Anal Chem. 2017;10:439–62. https://doi.org/10.1146/annurev-anchem-061516-045228.
Kalisky T, Quake SR. Single-cell genomics. Nat Methods. 2011;8:311.
Passarelli MK, Ewing AG. Single-cell imaging mass spectrometry. Curr Opin Chem Biol. 2013;17:854–9. https://doi.org/10.1016/j.cbpa.2013.07.017.
Rubakhin SS, Romanova EV, Nemes P, Sweedler JV. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8:S20–9. https://doi.org/10.1038/nmeth.1549.
Mathew AK, Padmanaban VC. Metabolomics: the apogee of the omics trilogy. Int J Pharm Pharm Sci. 2013;5:45–8.
Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739.
Patti GJ, Yanes O, Shriver LP, et al. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat Chem Biol. 2012;8:232.
Qiu S, Luo S, Evgrafov O, et al. Single-neuron RNA-Seq: technical feasibility and reproducibility. Front Genet. 2012;3:124.
Qiu S, Luo S, Evgrafov O, et al. Erratum: single-neuron RNA-Seq: technical feasibility and reproducibility. Front Genet. 2013;4:23.
Wang J, Fan HCC, Behr B, Quake SRR. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell. 2012;150:402–12. https://doi.org/10.1016/j.cell.2012.06.030.
Hou Y, Fan W, Yan L, et al. Genome analyses of single human oocytes. Cell. 2013;155:1492–506.
Tang F, Barbacioru C, Bao S, et al. Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell. 2010;6:468–78. https://doi.org/10.1016/j.stem.2010.03.015.
Xue Z, Huang K, Cai C, et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature. 2013;500:593–7. https://doi.org/10.1038/nature12364.
Yan L, Yang M, Guo H, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20:1131–9. https://doi.org/10.1038/nsmb.2660.
Guo H, Zhu P, Wu X, et al. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013;23:2126–35. https://doi.org/10.1101/gr.161679.113.
Kim J, Eberwine J. RNA: state memory and mediator of cellular phenotype. Trends Cell Biol. 2010;20:311–8.
Eberwine J, Sul JY, Bartfai T, Kim J. The promise of single-cell sequencing. Nat Methods. 2014;11:25–7. https://doi.org/10.1038/nmeth.2769.
Macaulay IC, Ponting CP, Voet T. Single-cell multiomics: multiple measurements from single cells. Trends Genet. 2017;33:155–68. https://doi.org/10.1016/j.tig.2016.12.003.
Yilmaz S, Singh AK. Single cell genome sequencing. Curr Opin Biotechnol. 2012;23:437.
Gross A, Schoendube J, Zimmermann S, et al. Technologies for single-cell isolation. Int J Mol Sci. 2015;16:16897.
Cheong R, Wang CJ, Levchenko A. Using a microfluidic device for high-content analysis of cell signaling. Sci Signal. 2009;2:pl2.
Binnig G, Rohrer H. Helvetica Phys. Acta 55, 726 (1982). Sci Am. 1985;253:50.
Garcia-Parajo MF. Optical antennas focus in on biology. Nat Photonics. 2008;2:201–3. https://doi.org/10.1038/nphoton.2008.37.
Amrania H, Drummond L, Coombes RC, et al. New IR imaging modalities for cancer detection and for intra-cell chemical mapping with a sub-diffraction mid-IR s-SNOM. Faraday Discuss. 2016;187:539. https://doi.org/10.1039/c5fd00150a.
Dereux A, Vigneron JP, Lambin P, Lucas AA. Theory of near-field optics with applications to SNOM and optical binding. Phys B Phys Condens Matter. 1991;175:65–7. https://doi.org/10.1016/0921-4526(91)90692-8.
Dürig U, Pohl DW, Rohner F. Near-field optical-scanning microscopy. J Appl Phys. 1986;59:3318. https://doi.org/10.1063/1.336848.
Abeyasinghe N, Kumar S, Sun K, et al. Enhanced emission from single isolated gold quantum dots investigated using two-photon-excited fluorescence near-field scanning optical microscopy. J Am Chem Soc. 2016;138:16299–307. https://doi.org/10.1021/jacs.6b07737.
Futamata M, Bruckbauer A. ATR-SNOM-Raman spectroscopy. Chem Phys Lett. 2001;341:425–30. https://doi.org/10.1016/S0009-2614(01)00545-0.
Butler HJ, Ashton L, Bird B, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016;11:664–87. https://doi.org/10.1038/nprot.2016.036.
Liu Z, Lavis LD, Betzig E. Imaging live-cell dynamics and structure at the single-molecule level. Mol Cell. 2015;58:644. https://doi.org/10.1016/j.molcel.2015.02.033.
Liang Z, Yin Z, Yang H, et al. Nanoscale surface analysis that combines scanning probe microscopy and mass spectrometry: a critical review. Trends Anal Chem. 2016;75:24–34. https://doi.org/10.1016/j.trac.2015.07.009.
Huth F, Govyadinov A, Amarie S, et al. Nano-FTIR absorption spectroscopy of molecular fingerprints at 20 nm spatial resolution. Nano Lett. 2012;12:3973. https://doi.org/10.1021/nl301159v.
Albella P, De La Osa RA, Moreno F, Maier SA. Electric and magnetic field enhancement with ultralow heat radiation dielectric nanoantennas: considerations for surface-enhanced spectroscopies. ACS Photon. 2014;1:524. https://doi.org/10.1021/ph500060s.
Della Picca F, Berte R, Rahmani M, et al. Tailored hypersound generation in single plasmonic nanoantennas. Nano Lett. 2016;16:1428. https://doi.org/10.1021/acs.nanolett.5b04991.
Regmi R, Winkler PM, Flauraud V, et al. Planar optical nanoantennas resolve cholesterol-dependent nanoscale heterogeneities in the plasma membrane of living cells. Nano Lett. 2017;17:6295. https://doi.org/10.1021/acs.nanolett.7b02973.
Drude P. Zur Elektronentheorie der Metalle; II. Teil. Galvanomagnetische und thermomagnetische Effecte. Ann Phys. 1900;3:369. https://doi.org/10.1002/andp.19003081102.
Sommerfeld A, Bethe H. Elektronentheorie der Metalle. In: Aufbau Der Zusammenhängenden Materie. New York, NY: Springer; 1933.
Sommerfeld A. Zur Elektronentheorie der Metalle auf Grund der Fermischen Statistik - I. Teil: Allgemeines. Zeitschrift für Physik. 1928;47:1–32. https://doi.org/10.1007/BF01391052.
Giannini V, Fernández-Domínguez AI, Heck SC, Maier SA. Plasmonic nanoantennas: fundamentals and their use in controlling the radiative properties of nanoemitters. Chem Rev. 2011;111:3888.
Wang AX, Kong X. Review of recent progress of plasmonic materials and nano-structures for surface-enhanced raman scattering. Materials (Basel). 2015;8:3024.
Pellegrotti JV, Acuna GP, Puchkova A, et al. Controlled reduction of photobleaching in DNA origami-gold nanoparticle hybrids. Nano Lett. 2014;14:2831. https://doi.org/10.1021/nl500841n.
Yuan H, Khatua S, Zijlstra P, et al. Thousand-fold enhancement of single-molecule fluorescence near a single gold nanorod. Angew Chem Int Ed. 2013;52:1217. https://doi.org/10.1002/anie.201208125.
Khatua S, Paulo PMR, Yuan H, et al. Resonant plasmonic enhancement of single-molecule fluorescence by individual gold nanorods. ACS Nano. 2014;8:4440. https://doi.org/10.1021/nn406434y.
Huth F, Chuvilin A, Schnell M, et al. Resonant antenna probes for tip-enhanced infrared near-field microscopy. Nano Lett. 2013;13:1065. https://doi.org/10.1021/nl304289g.
Puchkova A, Vietz C, Pibiri E, et al. DNA origami nanoantennas with over 5000-fold fluorescence enhancement and single-molecule detection at 25 μm. Nano Lett. 2015;15:8354–9.
Dulkeith E, Ringler M, Klar TA, et al. Gold nanoparticles quench fluorescence by phase induced radiative rate suppression. Nano Lett. 2005;5:585. https://doi.org/10.1021/nl0480969.
Etin AE, Yanik AA, Yilmaz C, et al. Monopole antenna arrays for optical trapping, spectroscopy, and sensing. Appl Phys Lett. 2011;98:98–101. https://doi.org/10.1063/1.3559620.
Jung I, Kim M, Kwak M, et al. Surface plasmon resonance extension through two-block metal-conducting polymer nanorods. Nat Commun. 2018;9:1010. https://doi.org/10.1038/s41467-018-03453-z.
Michieli N, Pilot R, Russo V, et al. Oxidation effects on the SERS response of silver nanoprism arrays. RSC Adv. 2017;7:369. https://doi.org/10.1039/c6ra26307k.
Kim M, Ko SM, Kim JM, et al. Dealloyed intra-nanogap particles with highly robust, quantifiable surface-enhanced Raman scattering signals for biosensing and bioimaging applications. ACS Cent Sci. 2018;4:277. https://doi.org/10.1021/acscentsci.7b00584.
Jin Q, Li M, Polat B, et al. Mechanical trap surface-enhanced raman spectroscopy for three-dimensional surface molecular imaging of single live cells. Angew Chem Int Ed. 2017;56:3822. https://doi.org/10.1002/anie.201700695.
Nair AK, Bhavitha KB, Perumbilavil S, et al. Multifunctional nitrogen sulfur co-doped reduced graphene oxide – Ag nano hybrids (sphere, cube and wire) for nonlinear optical and SERS applications. Carbon. 2018;132:380. https://doi.org/10.1016/j.carbon.2018.02.068.
Dill TJ, Rozin MJ, Brown ER, et al. Investigating the effect of Ag nanocube polydispersity on gap-mode SERS enhancement factors. Analyst. 2016;141:3916. https://doi.org/10.1039/c6an00212a.
Li Y, Ye Y, Fan Y, et al. Silver nanoprism-loaded eggshell membrane: a facile platform for in situ SERS monitoring of catalytic reactions. Crystals. 2017;7:45.
Pilipavicius J, Kaleinikaite R, Pucetaite M, et al. Controllable formation of high density SERS-active silver nanoprism layers on hybrid silica-APTES coatings. Appl Surf Sci. 2016;377:134. https://doi.org/10.1016/j.apsusc.2016.03.169.
Vitol EA, Orynbayeva Z, Friedman G, Gogotsi Y. Nanoprobes for intracellular and single cell surface-enhanced Raman spectroscopy (SERS). J Raman Spectrosc. 2012;43:817.
Altunbek M, Kuku G, Culha M. Gold nanoparticles in single-cell analysis for surface enhanced Raman scattering. Molecules. 2016;21(12):E1617.
Cowcher DP, Deckert-Gaudig T, Brewster VL, et al. Detection of protein glycosylation using tip-enhanced Raman scattering. Anal Chem. 2016;88:2105–12. https://doi.org/10.1021/acs.analchem.5b03535.
Xiao L, Wang H, Schultz ZD. Selective detection of RGD-integrin binding in cancer cells using tip enhanced raman scattering microscopy. Anal Chem. 2016;88:6547. https://doi.org/10.1021/acs.analchem.6b01344.
Balanis CA. Modern antenna handbook. New York, NY: Wiley; 2007.
Pozar DM. Microwave engineering. New York, NY: Wiley; 2012.
Bethe HA. Theory of diffraction by small holes. Phys Rev. 1944;66:163–82. https://doi.org/10.1103/PhysRev.66.163.
Bouwkamp CJ. Diffraction theory. Rep Prog Phys. 1954;17:35. https://doi.org/10.1088/0034-4885/17/1/302.
Levine H, Schwinger J. On the transmission coefficient of a circular aperture. Phys Rev. 1949;75:1608.
Drezet A, Woehl JC, Huant S. Diffraction by a small aperture in conical geometry: application to metal-coated tips used in near-field scanning optical microscopy. Phys Rev E Stat Phys Plasm Fluid Relat Interdiscip Top. 2002;65:046611. https://doi.org/10.1103/PhysRevE.65.046611.
Crouch GM, Han D, Bohn PW. Zero-mode waveguide nanophotonic structures for single molecule characterization. J Phys D Appl Phys. 2018;51:193001.
Napoli M, Eijkel JCT, Pennathur S. Nanofluidic technology for biomolecule applications: a critical review. Lab Chip. 2010;10:957.
Larkin J, Henley RY, Jadhav V, et al. Length-independent DNA packing into nanopore zero-mode waveguides for low-input DNA sequencing. Nat Nanotechnol. 2017;12:1169–75. https://doi.org/10.1038/nnano.2017.176.
Magde D, Elson E, Webb WW. Thermodynamic fluctuations in a reacting system measurement by fluorescence correlation spectroscopy. Phys Rev Lett. 1972;29:705. https://doi.org/10.1103/PhysRevLett.29.705.
Samiee KT, Foquet M, Guo L, et al. λ-repressor oligomerization kinetics at high concentrations using fluorescence correlation spectroscopy in zero-mode waveguides. Biophys J. 2005;88:2145. https://doi.org/10.1529/biophysj.104.052795.
Samiee KT, Moran-Mirabal JM, Cheung YK, Craighead HG. Zero mode waveguides for single-molecule spectroscopy on lipid membranes. Biophys J. 2006;90:3288. https://doi.org/10.1529/biophysj.105.072819.
Richards CI, Luong K, Srinivasan R, et al. Live-cell imaging of single receptor composition using zero-mode waveguide nanostructures. Nano Lett. 2012;12:3690–4. https://doi.org/10.1021/nl301480h.
Miyake T, Tanii T, Sonobe H, et al. Real-time imaging of single-molecule fluorescence with a zero-mode waveguide for the analysis of protein-protein interaction. Anal Chem. 2008;80:6018. https://doi.org/10.1021/ac800726g.
Zaino LP, Grismer DA, Han D, et al. Single occupancy spectroelectrochemistry of freely diffusing flavin mononucleotide in zero-dimensional nanophotonic structures. Faraday Discuss. 2015;8:535. https://doi.org/10.1039/c5fd00072f.
Gooding JJ, Gaus K. Single-molecule sensors: challenges and opportunities for quantitative analysis. Angew Chem Int Ed. 2016;55:11354–66. https://doi.org/10.1002/anie.201600495.
Murphy TW, Zhang Q, Naler LB, et al. Recent advances in the use of microfluidic technologies for single cell analysis. Analyst. 2018;143:60–80.
Song C, Tan SH. A perspective on the rise of optofluidics and the future. Micromachines. 2017;8:152.
Ma Z, Teo AJT, Tan SH, et al. Self-aligned interdigitated transducers for acoustofluidics. Micromachines. 2016;7:216. https://doi.org/10.3390/mi7120216.
TAG Optics Inc. TAG lens product family: the world’s fastest focusing lenses.
Blue-Scientific Optofluidics. http://www.blue-scientific.com/biological-afm-microscopy-jpk-instruments-bruker/. Accessed 1 Nov 2018.
Bedoya AC, Monat C, Domachuk P, et al. Measuring the dispersive properties of liquids using a microinterferometer. Appl Opt. 2011;50:2408. https://doi.org/10.1364/AO.50.002408.
Testa G, Persichetti G, Sarro PM, Bernini R. A hybrid silicon-PDMS optofluidic platform for sensing applications. Biomed Opt Express. 2014;5:417. https://doi.org/10.1364/BOE.5.000417.
Lapsley MI, Chiang IK, Zheng YB, et al. A single-layer, planar, optofluidic Mach-Zehnder interferometer for label-free detection. Lab Chip. 2011;11:1795. https://doi.org/10.1039/c0lc00707b.
Guo B, Lei C, Kobayashi H, et al. High-throughput, label-free, single-cell, microalgal lipid screening by machine-learning-equipped optofluidic time-stretch quantitative phase microscopy. Cytometry A. 2017;91:494. https://doi.org/10.1002/cyto.a.23084.
Lau AKS, Shum HC, Wong KKY, et al. Optofluidic time-stretch imaging – an emerging tool for high-throughput imaging flow cytometry. Lab Chip. 2016;16:1743. https://doi.org/10.1039/C5LC01458A.
Müller P, Schürmann M, Chan CJ, Guck J. Single-cell diffraction tomography with optofluidic rotation about a tilted axis. SPIE Proc. 2015;9548:95480U.
Lee KS, Lee KH, Kim SB, et al. Dynamic manipulation of particles via transformative optofluidic waveguides. Sci Rep. 2015;5:15170. https://doi.org/10.1038/srep15170.
Lee KH, Lee KS, Jung JH, et al. Optical mobility of blood cells for label-free cell separation applications. Appl Phys Lett. 2013;102:141911. https://doi.org/10.1063/1.4801951.
Schmidt H, Hawkins AR. Single-virus analysis through chip-based optical detection. Bioanalysis. 2016;8:867–70. https://doi.org/10.4155/bio-2016-0004.
Cai H, Parks JW, Wall TA, et al. Optofluidic analysis system for amplification-free, direct detection of Ebola infection. Sci Rep. 2015;5:14494. https://doi.org/10.1038/srep14494.
Pollnau M, Hammer M, Dongre C, Hoekstra HJWM. DNA separation and fluorescent detection in an optofluidic chip with sub-base-pair resolution. SPIE Proc. 2015;9320:93200J.
Knob R, Hanson RL, Tateoka OB, et al. Sequence-specific sepsis-related DNA capture and fluorescent labeling in monoliths prepared by single-step photopolymerization in microfluidic devices. J Chromatogr A. 2018;1562:12. https://doi.org/10.1016/j.chroma.2018.05.042.
Bertucci A, Manicardi A, Candiani A, et al. Detection of unamplified genomic DNA by a PNA-based microstructured optical fiber (MOF) Bragg-grating optofluidic system. Biosens Bioelectron. 2015;63:248. https://doi.org/10.1016/j.bios.2014.07.047.
Petras D, Jarmusch AK, Dorrestein PC. From single cells to our planet—recent advances in using mass spectrometry for spatially resolved metabolomics. Curr Opin Chem Biol. 2017;36:24–31. https://doi.org/10.1016/j.cbpa.2016.12.018.
Wikimedia Commons, Fyson D. Mass spectrometer schematic. 2008. https://commons.wikimedia.org/wiki/File:Mass_Spectrometer_Schematic.svg. Accessed 24 Jul 2018.
Mukhopadhyay SM. Sample preparation for microscopic and spectroscopic characterization of solid surfaces and films. In: Sample preparation techniques in analytical chemistry. Hoboken, NJ: John Wiley & Sons, Inc.; 2003.
Caprioli RM, Suter MJF. Continuous-flow fast atom bombardment: recent advances and applications. Int J Mass Spectrom Ion Process. 1992;118–119:449–76. https://doi.org/10.1016/0168-1176(92)85072-8.
Kralj B, Kramer V, Vrščaj V. Fast atom bombardment of molecules in the gaseous state. Int J Mass Spectrom Ion Phys. 1983;46:399–402. https://doi.org/10.1016/0020-7381(83)80136-3.
Takayama M. Gas-phase fast-atom bombardment mass spectrometry. Int J Mass Spectrom Ion Process. 1996;152:1–20. https://doi.org/10.1016/0168-1176(95)04298-9.
Fenn JB, Mann M, Meng CK, et al. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. https://doi.org/10.1126/science.2675315.
Tanaka K, Waki H, Ido Y, et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1988;2:151–3. https://doi.org/10.1002/rcm.1290020802.
Vertes A, Irinyi G, Gijbels R. Hydrodynamic model of matrix-assisted laser desorption mass spectrometry. Anal Chem. 1993;65:2389–93. https://doi.org/10.1021/ac00065a036.
Wu XW, Sadeghi M, Vertes A. Molecular dynamics of matrix-assisted laser desorption of leucine enkephalin guest molecules from nicotinic acid host crystal. J Phys Chem B. 1998;102:4770–8. https://doi.org/10.1021/jp9806361.
Sadeghi M, Wu X, Vertes A. Conformation changes, complexation, and phase transition in matrix-assisted laser desorption. J Phys Chem B. 2001;105:2578–87. https://doi.org/10.1021/jp0032715.
Stolee JA, Walker BN, Zorba V, et al. Laser–nanostructure interactions for ion production. Phys Chem Chem Phys. 2012;14:8453. https://doi.org/10.1039/c2cp00038e.
Karas M, Bachmann D, Hillenkamp F. Influence of the wavelength in high-irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal Chem. 1985;57:2935–9. https://doi.org/10.1021/ac00291a042.
Benninghoven A, Rudenauer FG, Werner HW. Secondary ion mass spectrometry: basic concepts, instrumental aspects, applications and trends. New York, NY: John Wiley & Sons; 1987.
Perkel JM. LIFE SCIENCE TECHNOLOGIES: mass spec imaging: from bench to bedside. Science. 2013;340:1119–21. https://doi.org/10.1126/science.opms.p1300076.
McEwen CN, McKay RG. A combination atmospheric pressure LC/MS:GC/MS ion source: advantages of dual AP-LC/MS:GC/MS instrumentation. J Am Soc Mass Spectrom. 2005;16:1730–8. https://doi.org/10.1016/j.jasms.2005.07.005.
McEwen CN, McKay RG, Larsen BS. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal Chem. 2005;77:7826–31. https://doi.org/10.1021/ac051470k.
Knochenmuss R, Zenobi R. MALDI ionization: the role of in-plume processes. Chem Rev. 2003;103:441–52. https://doi.org/10.1021/cr0103773.
Knochenmuss R. A quantitative model of ultraviolet matrix-assisted laser desorption/ionization including analyte ion generation. Anal Chem. 2003;75:2199–207. https://doi.org/10.1021/ac034032r.
Laiko VV, Baldwin MA, Burlingame AL. Atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2000;72:652–7. https://doi.org/10.1021/ac990998k.
Cho Y-T, Su H, Wu W-J, et al. Biomarker characterization by MALDI–TOF/MS. In: Advances in clinical chemistry. Amsterdam: Elsevier; 2015. p. 209–54.
Dale MJ, Knochenmuss R, Zenobi R. Graphite/liquid mixed matrices for laser desorption/ionization mass spectrometry. Anal Chem. 1996;68:3321–9. https://doi.org/10.1021/ac960558i.
Vertes A. Soft laser desorption ionization - Maldi, dios and nanostructures. Springer Ser Opt Sci. 2006;129:505–28. https://doi.org/10.1007/978-0-387-30453-3_20.
Colaianni L, Kung SC, Taggart DK, et al. Laser desorption ionization-mass spectrometry detection of amino acids and peptides promoted by gold nanowires. Sens Lett. 2010;8:539–44. https://doi.org/10.1166/sl.2010.1308.
Nayak R, Knapp DR. Matrix-free LDI mass spectrometry platform using patterned nanostructured gold thin film. Anal Chem. 2010;82:7772–8. https://doi.org/10.1021/ac1017277.
Pyayt AL, Wiley B, Xia Y, et al. Integration of photonic and silver nanowire plasmonic waveguides. Nat Nanotechnol. 2008;3:660–5. https://doi.org/10.1038/nnano.2008.281.
Seino T, Sato H, Yamamoto A, et al. Matrix-free laser desorption/ionization-mass spectrometry using self-assembled germanium nanodots. Anal Chem. 2007;79:4827–32. https://doi.org/10.1021/ac062216a.
Sato H, Nemoto A, Yamamoto A, Tao H. Surface cleaning of germanium nanodot ionization substrate for surface-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:603–10.
Kawasaki H, Yonezawa T, Watanabe T, Arakawa R. Platinum nanoflowers for surface-assisted laser desorption/ionization mass spectrometry of biomolecules. J Phys Chem C. 2007;111:16278–83.
Cha S, Yeung ES. Colloidal graphite-assisted laser desorption/ionization mass spectrometry and MS n of small molecules. 1. Imaging of cerebrosides directly from rat brain tissue. Anal Chem. 2007;79:2373–85.
Kang M, Pyun J, Lee J, et al. Nanowire-assisted laser desorption and ionization mass spectrometry for quantitative analysis of small molecules. Rapid Commun Mass Spectrom. 2005;19:3166–70.
Go EP, Apon JV, Luo G, et al. Desorption/ionization on silicon nanowires. Anal Chem. 2005;77:1641–6.
Chen Y, Vertes A. Adjustable fragmentation in laser desorption/ionization from laser-induced silicon microcolumn arrays. Nature. 2006;78:5835–44.
Northen TR, Yanes O, Northen MT, et al. Clathrate nanostructures for mass spectrometry. Nature. 2007;449:1033–6. https://doi.org/10.1038/nature06195.
Wei J, Buriak JM, Siuzdak G. Desorption – ionization mass spectrometryonporoussilicon. Nature. 1999;399:243–6. https://doi.org/10.1038/20400.
Walker BN, Stolee JA, Pickel DL, et al. Tailored silicon nanopost arrays for resonant nanophotonic ion production. J Phys Chem C. 2010;114:4835–40. https://doi.org/10.1021/jp9110103.
Walker BN, Stolee JA, Vertes A. Nanophotonic ionization for ultratrace and single-cell analysis by mass spectrometry. Anal Chem. 2012;84:7756–62. https://doi.org/10.1021/ac301238k.
Korte AR, Stopka SA, Morris N, et al. Large-scale metabolite analysis of standards and human serum by laser desorption ionization mass spectrometry from silicon nanopost arrays. Anal Chem. 2016;88:8989–96. https://doi.org/10.1021/acs.analchem.6b01186.
Stopka SA, Holmes XA, Korte AR, et al. Trace analysis and reaction monitoring by nanophotonic ionization mass spectrometry from elevated bow-tie and silicon nanopost arrays. Adv Funct Mater. 2018;28:1801730. https://doi.org/10.1002/adfm.201801730.
Wang Y, Ta VD, Gao Y, et al. Stimulated emission and lasing from CdSe/CdS/ZnS core-multi-shell quantum dots by simultaneous three-photon absorption. Adv Mater. 2014;26:2954. https://doi.org/10.1002/adma.201305125.
Zhu H, Fu Y, Meng F, et al. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat Mater. 2015;14:636. https://doi.org/10.1038/nmat4271.
Huang MH, Mao S, Feick H, et al. Room-temperature ultraviolet nanowire nanolasers. Science. 2001;292:1897. https://doi.org/10.1126/science.1060367.
Galanzha EI, Weingold R, Nedosekin DA, et al. Spaser as a biological probe. Nat Commun. 2017;8:15528.
Li L, Ge J, Wu H, et al. Organelle-specific detection of phosphatase activities with two-photon fluorogenic probes in cells and tissues. J Am Chem Soc. 2012;134:12157. https://doi.org/10.1021/ja3036256.
Huang C, Wang K, Yang Z, et al. Up-conversion perovskite nanolaser with single mode and low threshold. J Phys Chem C. 2017;121:10071. https://doi.org/10.1021/acs.jpcc.7b00875.
Li M, Zhi M, Zhu H, et al. Ultralow-threshold multiphoton-pumped lasing from colloidal nanoplatelets in solution. Nat Commun. 2015;6:8513. https://doi.org/10.1038/ncomms9513.
Baba T. Biosensing using photonic crystal nanolasers. MRS Commun. 2015;5:555. https://doi.org/10.1557/mrc.2015.73.
Kita S, Hachuda S, Otsuka S, et al. Super-sensitivity in label-free protein sensing using a nanoslot nanolaser. Opt Express. 2011;19:17683. https://doi.org/10.1364/OE.19.017683.
Alix-Panabières C, Pantel K. Biological labels: here comes the spaser. Nat Mater. 2017;16:790.
Solowan H-P, Kryschi C. Facile design of a plasmonic nanolaser. Condens Matter. 2017;2:8.
Lane LA, Smith AM, Lian T, Nie S. Compact and blinking-suppressed quantum dots for single-particle tracking in live cells. J Phys Chem B. 2014;118:14140. https://doi.org/10.1021/jp5064325.
Sabaeian M, Khaledi-Nasab A. Size-dependent intersubband optical properties of dome-shaped InAs/GaAs quantum dots with wetting layer. Appl Opt. 2012;51:4176–85. https://doi.org/10.1364/AO.51.004176.
Khaledi-Nasab A, Sabaeian M, Sahrai M, Fallahi V. Kerr nonlinearity due to intersubband transitions in a three-level InAs/GaAs quantum dot: the impact of a wetting layer on dispersion curves. J Opt. 2014;16:55004.
Walling MA, Novak JA, Shepard JRE. Quantum dots for live cell and in vivo imaging. Int J Mol Sci. 2009;10:441–91. https://doi.org/10.3390/ijms10020441.
Pelley JL, Daar AS, Saner MA. State of academic knowledge on toxicity and biological fate of quantum dots. Toxicol Sci. 2009;112:276–96. https://doi.org/10.1093/toxsci/kfp188.
Choi HS, Liu W, Misra P, et al. Renal clearance of nanoparticles. Nat Biotechnol. 2007;25:1165–70. https://doi.org/10.1038/nbt1340.
Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969. https://doi.org/10.1038/nbt994.
Yang Y, Jing L, Yu X, et al. Coating aqueous quantum dots with silica via reverse microemulsion method: toward size-controllable and robust fluorescent nanoparticles. Chem Mater. 2007;19:4123. https://doi.org/10.1021/cm070798m.
Thoniyot P, Tan MJ, Karim AA, et al. Nanoparticle–hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv Sci. 2015;2:1.
Ma Y, Wang M, Li W, et al. Live cell imaging of single genomic loci with quantum dot-labeled TALEs. Nat Commun. 2017;8:15318. https://doi.org/10.1038/ncomms15318.
Qiu Y, Zhou B, Yang X, et al. Novel single-cell analysis platform based on a solid-state zinc-coadsorbed carbon quantum dots electrochemiluminescence probe for the evaluation of CD44 expression on breast cancer cells. ACS Appl Mater Interfaces. 2017;9:16848. https://doi.org/10.1021/acsami.7b02793.
Katrukha EA, Mikhaylova M, Van Brakel HX, et al. Probing cytoskeletal modulation of passive and active intracellular dynamics using nanobody-functionalized quantum dots. Nat Commun. 2017;8:14772. https://doi.org/10.1038/ncomms14772.
Fang L, Ohfuji H, Irifune T. A novel technique for the synthesis of nanodiamond powder. J Nanomater. 2013;2013:41. https://doi.org/10.1155/2013/201845.
Holt KB. Diamond at the nanoscale: applications of diamond nanoparticles from cellular biomarkers to quantum computing. Philos Trans R Soc A Math Phys Eng Sci. 2007;365:2845. https://doi.org/10.1098/rsta.2007.0005.
Hsiao WWW, Hui YY, Tsai PC, Chang HC. Fluorescent nanodiamond: a versatile tool for long-term cell tracking, super-resolution imaging, and nanoscale temperature sensing. Acc Chem Res. 2016;49:400.
Lin H-H, Lee H-W, Lin R-J, et al. Tracking and finding slow-proliferating/quiescent cancer stem cells with fluorescent nanodiamonds. Small. 2015;11:4394–402. https://doi.org/10.1002/smll.201500878.
Liu KK, Wang CC, Cheng CL, Chao JI. Endocytic carboxylated nanodiamond for the labeling and tracking of cell division and differentiation in cancer and stem cells. Biomaterials. 2009;30:4249. https://doi.org/10.1016/j.biomaterials.2009.04.056.
Hui YY, Su LJ, Chen OY, et al. Wide-field imaging and flow cytometric analysis of cancer cells in blood by fluorescent nanodiamond labeling and time gating. Sci Rep. 2014;4:5574. https://doi.org/10.1038/srep05574.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Nisar, M.S., Zhao, X. (2021). Nanophotonic Techniques for Single-Cell Analysis. In: Zhao, X., Lu, M. (eds) Nanophotonics in Biomedical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-6137-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-6137-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6136-8
Online ISBN: 978-981-15-6137-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)