Abstract
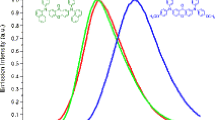
9,10-Bis(4-methoxyphenyl) anthracene (2a) and 9,10-bis(4-formylphenyl) anthracene (2b) were synthesized by Suzuki coupling reaction. The target molecular structure was fully characterized by 1H NMR and mass spectra. The photophysical properties of two compounds in diluted CH2Cl2 solutions were obtained by using UV-vis and FL spectra and the thermal stability were researched by TGA and DSC. At the same time, the aided verification was operating by DFT calculations. The results show that two compounds have excellent thermal stability because of the increase of substituents which can benefit to improve thermal stability. The decomposition temperatures (Td) of 9,10-bis(4-methoxyphenyl) anthracene can reach to 307 °C. All these molecules show highly blue emissions (λmaxFL < 477 nm) with excellent quantum yields (Φf > 0.52) in solution and with the increase of π-conjugation, the emission peak maximum exhibits the red Stokes shift (40 nm). The UV-vis absorption and FL emission spectra of compound 2a have almost no change depending on the solvent polarity, moreover, the FL emission spectra of compound 2b has the obvious red Stokes shift on moving from non-polar solvent(cyclohexane) to strong-polar solvent(DMF). That can be utilized as promising potential blue emitters in organic light-emitting devices (OLEDs) applications.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Chen JX, Huang XW (2011) OLED display: material and device. Posts and Telecom Press, Beijing
Huang W, Mi BX, Gao ZQ (2011) Organic electronics. Science Press, Beijing
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492:234–238
Du JR, Wang MR, Chen NK, Xie SY, Yu HM, Wu Q (2016) Instability origin and improvement scheme of facial Alq3 for blue OLED application. Chem. Res. Chinese Universities 32(3):423–427
Li NQ, Fan ZK, Zhao HR, Quan YW, Chen QM, Ye SH, Li SH, Fan QL, Huang W (2016) A bipolar macrospirocyclic oligomer based on triphenylamine and 4,5-diazafluorene as a solution-processable host for blue phosphorescent organic light-emitting diodes. Dyes Pigments 134:348–357
Shao SY, Ding JQ, Wang LX (2016) New applications of poly(arylene ether)s in organic light-emitting diodes. Chin Chem Lett 27:1201–1208
Tanaka Y, Takahashi T, Nishide J, Hiraga Y, Nakanotani H, Adachi C (2016) Application of wide-energy-gap material 3,4-di(9H-carbazol-9-yl)benzonitrile in organic light-emitting diodes. Thin Solid Films 619:120–124
Yu MQ, Wang SM, Shao SY, Ding JQ, Wang LX, Jing XB, Wang FS (2015) Starburst 4,4′,4″-tris(carbazol-9-yl)-triphenylamine-based deep-blue fluorescent emitters with tunable oligophenyl length for solution-processed undoped organic light-emitting diodes. J Mater Chem C 3:861–869
Mallesham G, Balaiah S, Ananth Reddy M, Sridhar B, Punita S, et al. (2014) Design and synthesis of novel anthracene derivatives as n-type emitters for electroluminescent devices: a combined experimental and DFT study. Photoch Photobio Sci 13:342–357
Zhao L, Wang SM, Ding JQ, Wang LX (2016) Synthesis and characterization of solution-processible anthracene-based deep-blue fluorescent dendrimers. Chin Sci Bull 61:325–332
Sun J, Zhong HL, Xu EJ, Zeng DL, Zhang JH, Xu HG, Zhu WQ, Fang Q (2010) An X-shaped solution-processible oligomer having an anthracene unit as a core: a new organic light-emitting material with high thermostability and efficiency. Org Electron 11:74–80
Kang I, Back JY, Kim R, Kim YH, Kwon SK (2012) High efficient and high color pure blue light emitting materials: new asymmetrically highly twisted host and guest based on anthracene. Dyes Pigm 92:588–595
Pope M, Kallmann H, Magnante P (1963) Electroluminescence in organic crystals. Chem Phys 38:2042–2043
Zhang W, Wang Q, Feng X, Yang L, Wu YK, Wei XF (2017) Anthracene-based derivatives: synthesis, photophysical properties and electrochemical properties. Chem Res Chin Univ 33(4):603–610
Venkataramana G, Sankararaman S (2006) Synthesis and spectroscopic investigation of aggregation through cooperative π–π and C–H···O interactions in a novel pyrene octaaldehyde derivative. Org Lett 8:2739–2742
Sciano JC (1989) Handbook of organic photochemistry. CRC Press, Boca Raton, FL
Yucel B, Meral K, Ekinci D, Uzunoglu GY, et al (2014) Synthesis and characterization of solution processable 6,11-dialkynyl substituted indeno[1,2-b]anthracenes. Dyes Pigm 100:104–117
Acknowledgements
This study is funded by the Scientific Research Common Program of Beijing Municipal Commission of Education of China (Nos. KM201810015012, KM201810015003).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Zhang, W., Wu, Y., Feng, X., Xu, Y., Ma, L., Wei, X. (2020). Synthesis and Photophysical Properties of 9,10-Diarylanthracene. In: Zhao, P., Ye, Z., Xu, M., Yang, L. (eds) Advanced Graphic Communication, Printing and Packaging Technology. Lecture Notes in Electrical Engineering, vol 600. Springer, Singapore. https://doi.org/10.1007/978-981-15-1864-5_110
Download citation
DOI: https://doi.org/10.1007/978-981-15-1864-5_110
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1863-8
Online ISBN: 978-981-15-1864-5
eBook Packages: EngineeringEngineering (R0)