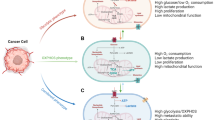
Abstract
Metabolic dysfunctionality is undoubtedly the central phenomenon that marks the onset of malignancy in human body. Various hormones particularly adipokines work collectively with a purpose to maintain metabolic homeostasis in cells; however, metabolic disruptions occur as a result of up- and downregulation of metabolic elements that ultimately pave way toward cancer onset. In cancer cell metabolism, hypoxic environment along with upregulation of various oncogenes enables the metabolic transformation of tumor cells. Tumor cells undergo shift from normal metabolic pathways and adopt unique metabolic patterns, relying primarily upon aerobic glycolysis or “Warburg effect” to meet their energy requirements. Studies have also validated the role of mutated metabolic enzymes whose over- or under-expression leads to metabolic reprogramming of cancer cells. Meanwhile, a major bulk of cancer related research is focused upon “cancer stem cells” as they pose maximum resistance toward chemotherapeutic drugs. The current chapter sequentially discusses various cancer associated-metabolic mechanisms, drug resistance patterns, and functioning of various regulatory genes (oncogenes and anti-oncogenes) associated with metastasis, followed by a brief account of various therapeutic and dietary options which have validated potential in confronting these metabolic changes and, in turn, impeding cancer growth and proliferation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Balaban RS (2006) Maintenance of the metabolic homeostasis of the heart. Ann N Y Acad Sci 1080(1):140–153
Wilson DF (2017) Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. J Physiol 595(23):7023–7038
Chance B, Williams G (1955) Respiratory enzymes in oxidative phosphorylation III. The steady state. J Biol Chem 217(1):409–428
Wu J (2017) New ways to maintain or disrupt metabolic homeostasis. J Mol Cell Biol 9(5):351–351. https://doi.org/10.1093/jmcb/mjx049
Arner P, Bernard S, Salehpour M, Possnert G, Liebl J, Steier P, Buchholz BA, Eriksson M, Arner E, Hauner H (2011) Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478(7367):110
Allison MB, Myers MG Jr (2014) Connecting leptin signaling to biological function. J Endocrinol 223(1):T25
Lumeng CN, Saltiel AR (2011) Inflammatory links between obesity and metabolic disease. J Clin Invest 121(6):2111–2117
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11(2):85
Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17(1):55
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112(1):91–100
Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23(5):770–784
Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu Y-H, Kozlova A, Voss H, Martins GG (2015) Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163(1):84–94
Eldor R, Raz I (2006) Lipotoxicity versus adipotoxicity—the deleterious effects of adipose tissue on beta cells in the pathogenesis of type 2 diabetes. Diabetes Res Clin Pract 74(2):S3–S8
Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H (2003) Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422(6928):173
Palmer BF, Clegg DJ (2014) Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol 397(1-2):51–58
Yingzhong Y, Droma Y, Rili G, Kubo K (2006) Regulation of body weight by leptin, with special reference to hypoxia-induced regulation. Intern Med 45(16):941–946
Kaufman RJ, Scheuner D, Schröder M, Shen X, Lee K, Liu CY, Arnold SM (2002) The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol 3(6):411
Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17(4):491–506
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251
Kim H-J, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang J-T, Park JH, Yang HJ, Kim M-S, Kwon DY (2010) Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res 10(2):722–731
Mollica MP, Iossa S, Liverini G, Soboll S (1998) Steady state changes in mitochondrial electrical potential and proton gradient in perfused liver from rats fed a high fat diet. Mol Cell Biochem 178(1-2):213–217
Qiu H, Schlegel V (2018) Impact of nutrient overload on metabolic homeostasis. Nutr Rev 76(9):693–707
Laybutt D, Preston A, Åkerfeldt M, Kench J, Busch A, Biankin A, Biden T (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50(4):752–763
Widdowson EM (1976) The response of the sexes to nutritional stress. Proc Nutr Soc 35(2):175–180
Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S (2002) Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 51(9):2734–2741
Mauvais-Jarvis F (2015) Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6(1):14
Nelson DL, Lehninger AL, Cox MM (2008) Lehninger principles of biochemistry. Macmillan, London
Warburg O (1925) The metabolism of carcinoma cells. J Cancer Res 9(1):148–163
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10:671. https://doi.org/10.1038/nrd3504
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9(6):425–434
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM (2015) An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162(3):540–551
Crabtree HG (1929) Observations on the carbohydrate metabolism of tumours. Biochem J 23(3):536
Bonora E, Porcelli AM, Gasparre G, Biondi A, Ghelli A, Carelli V, Baracca A, Tallini G, Martinuzzi A, Lenaz G (2006) Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res 66(12):6087–6096
López-Lázaro M (2008) The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anti Cancer Agents Med Chem 8(3):305–312
Ikebuchi Y, Masumoto N, Tasaka K, Koike K, Kasahara K, Miyake A, Tanizawa O (1991) Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem 266(20):13233–13237
Erecińska M, Deas J, Silver I (1995) The effect of pH on glycolysis and phosphofructokinase activity in cultured cells and synaptosomes. J Neurochem 65(6):2765–2772
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3(3):187–197
Semenza GL (2006) Development of novel therapeutic strategies that target HIF-1. Expert Opin Ther Targets 10(2):267–280
Wu C-A, Chao Y, Shiah S-G, Lin W-W (2013) Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Mol Cell Res 1833(5):1147–1156
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 17(1):113–124
Svensson RU, Shaw RJ (2012) Cancer metabolism: tumour friend or foe. Nature 485(7400):590
Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B (2010) The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 9(10):1960–1971
Lu J, Tan M, Cai Q (2015) The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356(2):156–164
Hammoudi N, Ahmed KBR, Garcia-Prieto C, Huang P (2011) Metabolic alterations in cancer cells and therapeutic implications. Chin J Cancer 30(8):508
Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29(5):625
Ramanathan A, Wang C, Schreiber SL (2005) Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci 102(17):5992–5997
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC (2009b) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274):739
Vander Heiden MG, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology. Cell 168(4):657–669
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16(10):619
Eales K, Hollinshead K, Tennant D (2016) Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5(1):e190
Dang CV (2010) Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res 70(3):859–862
Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H (2012) Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 15(1):110–121
Dang CV, Le A, Gao P (2009a) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15(21):6479–6483. https://doi.org/10.1158/1078-0432.ccr-09-0889
Mullen AR, Wheaton WW, Jin ES, Chen P-H, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ (2011) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481:385. https://doi.org/10.1038/nature10642
Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, Chandel NS, DeBerardinis RJ (2014) Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 7(5):1679–1690. https://doi.org/10.1016/j.celrep.2014.04.037
Rankin EB, Giaccia AJ (2016) Hypoxic control of metastasis. Science 352(6282):175–180. https://doi.org/10.1126/science.aaf4405
Benjamin DI, Cravatt BF, Nomura DK (2012) Global profiling strategies for mapping dysregulated metabolic pathways in cancer. Cell Metab 16(5):565–577. https://doi.org/10.1016/j.cmet.2012.09.013
Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA (2016) Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541:41. https://doi.org/10.1038/nature20791
Nath A, Li I, Roberts LR, Chan C (2015) Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep 5:14752–14752. https://doi.org/10.1038/srep14752
Nomura DK, Long JZ, Niessen S, Hoover HS, Ng S-W, Cravatt BF (2010) Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell 140(1):49–61. https://doi.org/10.1016/j.cell.2009.11.027
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol 19(1):17–24. https://doi.org/10.1016/j.semcancer.2008.11.006
Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25(34):4777–4786. https://doi.org/10.1038/sj.onc.1209603
Bustamante E, Pedersen PL (1980) Mitochondrial hexokinase of rat hepatoma cells in culture: solubilization and kinetic properties. Biochemistry 19(22):4972–4977. https://doi.org/10.1021/bi00563a006
Bustamante E, Morris HP, Pedersen PL (1981) Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem 256(16):8699–8704
Biaglow JE, Miller RA (2005) The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther 4(1):6–13. https://doi.org/10.4161/cbt.4.1.1434
Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17(1):72–79. https://doi.org/10.1634/theoncologist.2011-0386
Kosmider O, Gelsi-Boyer V, Slama L, Dreyfus F, Beyne-Rauzy O, Quesnel B, Hunault-Berger M, Slama B, Vey N, Lacombe C, Solary E, Birnbaum D, Bernard OA, Fontenay M (2010) Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia 24(5):1094–1096. https://doi.org/10.1038/leu.2010.52
Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med 361(11):1058–1066. https://doi.org/10.1056/NEJMoa0903840
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. https://doi.org/10.1126/science.1164382
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von Deimling A (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118(4):469–474. https://doi.org/10.1007/s00401-009-0561-9
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8):765–773. https://doi.org/10.1056/NEJMoa0808710
Losman JA, Kaelin WG Jr (2013) What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev 27(8):836–852. https://doi.org/10.1101/gad.217406.113
Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, Leung IK, Li XS, Woon EC, Yang M, McDonough MA, King ON, Clifton IJ, Klose RJ, Claridge TD, Ratcliffe PJ, Schofield CJ, Kawamura A (2011) The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 12(5):463–469. https://doi.org/10.1038/embor.2011.43
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19(1):17–30. https://doi.org/10.1016/j.ccr.2010.12.014
Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, Cornelisse CJ, Devilee P, Devlin B (2000) Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287(5454):848–851. https://doi.org/10.1126/science.287.5454.848
Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, Lai AH, Kelly L, Hornick JL, O’Sullivan M, de Krijger RR, Dinjens WN, Demetri GD, Antonescu CR, Fletcher JA, Helman L, Stratakis CA (2011) Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A 108(1):314–318. https://doi.org/10.1073/pnas.1009199108
Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, Maher ER (2008) Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 100(17):1260–1262. https://doi.org/10.1093/jnci/djn254
Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z (2005) Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121(7):1043–1057. https://doi.org/10.1016/j.cell.2005.05.025
Brière J-J, Favier J, Bénit P, Ghouzzi VE, Lorenzato A, Rabier D, Di Renzo MF, Gimenez-Roqueplo A-P, Rustin P (2005) Mitochondrial succinate is instrumental for HIF1α nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Hum Mol Genet 14(21):3263–3269. https://doi.org/10.1093/hmg/ddi359
Michalowska I, Cwikla J, Prejbisz A, Kwiatek P, Szperl M, Michalski W, Wyrwicz L, Kusmierczyk M, Januszewicz A, Maciejczyk A, Roszczynko M, Peczkowska M (2016) Mediastinal paragangliomas related to SDHx gene mutations. Kardiochir Torakochirurgia Pol 13(3):276–282. https://doi.org/10.5114/kitp.2016.62624
Bayley JP, van Minderhout I, Weiss MM, Jansen JC, Oomen PH, Menko FH, Pasini B, Ferrando B, Wong N, Alpert LC, Williams R, Blair E, Devilee P, Taschner PE (2006) Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet 7:1. https://doi.org/10.1186/1471-2350-7-1
Kuroda N, Yorita K, Nagasaki M, Harada Y, Ohe C, Jeruc J, Raspollini MR, Michal M, Hes O, Amin MB (2016) Review of succinate dehydrogenase-deficient renal cell carcinoma with focus on clinical and pathobiological aspects. Pol J Pathol 67(1):3–7
Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peçzkowska M, Morrison CD, Lehtonen R, Januszewicz A, Järvinen H, Juhola M (2004) Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 74(1):153–159
Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW (1999) PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet 21(1):99–102. https://doi.org/10.1038/5042
Broderick DK, Di C, Parrett TJ, Samuels YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD, Yan H (2004) Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res 64(15):5048–5050. https://doi.org/10.1158/0008-5472.can-04-1170
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304(5670):554. https://doi.org/10.1126/science.1096502
Kang S, Bader AG, Vogt PK (2005) Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A 102(3):802–807. https://doi.org/10.1073/pnas.0408864102
Nguyen LV, Vanner R, Dirks P, Eaves CJ (2012) Cancer stem cells: an evolving concept. Nat Rev Cancer 12(2):133–143. https://doi.org/10.1038/nrc3184
Jackson EB, Brues AM (1941) Studies on a transplantable embryoma of the mouse. Cancer Res 1(6):494–498
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648. https://doi.org/10.1038/367645a0
Easwaran H, Tsai HC, Baylin SB (2014) Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell 54(5):716–727. https://doi.org/10.1016/j.molcel.2014.05.015
Wu Z, Wei D, Gao W, Xu Y, Hu Z, Ma Z, Gao C, Zhu X, Li Q (2015) TPO-induced metabolic reprogramming drives liver metastasis of colorectal cancer CD110+ tumor-initiating cells. Cell Stem Cell 17(1):47–59. https://doi.org/10.1016/j.stem.2015.05.016
Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, Baba H, Saya H, Nagano O (2010) CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 101(3):673–678. https://doi.org/10.1111/j.1349-7006.2009.01430.x
Dean M (2009) ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia 14(1):3–9. https://doi.org/10.1007/s10911-009-9109-9
Meyer MJ, Fleming JM, Lin AF, Hussnain SA, Ginsburg E, Vonderhaar BK (2010) CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res 70(11):4624–4633. https://doi.org/10.1158/0008-5472.can-09-3619
Schober M, Fuchs E (2011) Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A 108(26):10544–10549. https://doi.org/10.1073/pnas.1107807108
Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE (2011) Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A 108(16):6468–6473. https://doi.org/10.1073/pnas.1005529108
Nowell PC (1986) Mechanisms of tumor progression. Cancer Res 46(5):2203–2207
Baylin SB, Jones PA (2011) A decade of exploring the cancer epigenome – biological and translational implications. Nat Rev Cancer 11:726. https://doi.org/10.1038/nrc3130
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329. https://doi.org/10.1038/nm.2328
Dick JE (2008) Stem cell concepts renew cancer research. Blood 112(13):4793–4807. https://doi.org/10.1182/blood-2008-08-077941
Shackleton M, Quintana E, Fearon ER, Morrison SJ (2009) Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138(5):822–829. https://doi.org/10.1016/j.cell.2009.08.017
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM (2007) Identification of pancreatic cancer stem cells. Cancer Res 67(3):1030–1037. https://doi.org/10.1158/0008-5472.can-06-2030
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3(7):730–737
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445(7123):106–110. https://doi.org/10.1038/nature05372
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. https://doi.org/10.1038/nature03128
Batlle E, Clevers H (2017) Cancer stem cells revisited. Nat Med 23(10):1124–1134. https://doi.org/10.1038/nm.4409
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES (2011) Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 146(4):633–644. https://doi.org/10.1016/j.cell.2011.07.026
Peiris-Pagès M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP (2016) Cancer stem cell metabolism. Breast Cancer Res 18(1):55–55. https://doi.org/10.1186/s13058-016-0712-6
Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, Reue K, Christofk H, Mischel PS, Pajonk F (2011) Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A 108(38):16062–16067. https://doi.org/10.1073/pnas.1106704108
Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA (2017) Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541(7635):41–45. https://doi.org/10.1038/nature20791
Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, Stevens B, Pei S, Balys M, Ashton JM, Klemm DJ, Woolthuis CM, Stranahan AW, Park CY, Jordan CT (2016) Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell 19(1):23–37. https://doi.org/10.1016/j.stem.2016.06.001
Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton Valerie G, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T (2017) The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19:518. https://doi.org/10.1038/ncb3513
Clarke MF, Hass AT (2006) Cancer stem cells. In: Reviews in cell biology and molecular medicine. Wiley, Hoboken
Dang CV (2012) MYC on the path to cancer. Cell 149(1):22–35. https://doi.org/10.1016/j.cell.2012.03.003
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21(3):297–308. https://doi.org/10.1016/j.ccr.2012.02.014
Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D (1997) Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 57(22):4997–5000
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454(7205):776–779. https://doi.org/10.1038/nature07091
Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541. https://doi.org/10.1038/onc.2008.247
Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP (2010) Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42(5):454–458. https://doi.org/10.1038/ng.556
Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, Carracedo A, Vander Heiden MG, Cantley LC, Pinton P, Haigis MC, Pandolfi PP (2012) Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149(1):49–62. https://doi.org/10.1016/j.cell.2012.02.030
Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441(7092):424–430. https://doi.org/10.1038/nature04869
Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL (2004) Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 18(13):1533–1538. https://doi.org/10.1101/gad.1199104
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115(5):577–590
Muller PA, Vousden KH (2013) p53 mutations in cancer. Nat Cell Biol 15(1):2–8. https://doi.org/10.1038/ncb2641
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431. https://doi.org/10.1016/j.cell.2009.04.037
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126(1):107–120. https://doi.org/10.1016/j.cell.2006.05.036
Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E (2004) The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64(7):2627–2633
Zhang C, Liu J, Wu R, Liang Y, Lin M, Liu J, Chan CS, Hu W, Feng Z (2014) Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget 5(14):5535–5546. https://doi.org/10.18632/oncotarget.2137
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X (2011) p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 13(3):310–316. https://doi.org/10.1038/ncb2172
Tavana O, Gu W (2017) Modulation of the p53/MDM2 interplay by HAUSP inhibitors. J Mol Cell Biol 9(1):45–52. https://doi.org/10.1093/jmcb/mjw049
Zhou G, Pantelopulos GA, Mukherjee S, Voelz VA (2017) Bridging microscopic and macroscopic mechanisms of p53-MDM2 binding with kinetic network models. Biophys J 113(4):785–793. https://doi.org/10.1016/j.bpj.2017.07.009
Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, Koochekpour S, Saleem M, Huang H, Lu J, Deng Y (2014) Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep 8(5):1461–1474. https://doi.org/10.1016/j.celrep.2014.07.053
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124(2):315–329. https://doi.org/10.1016/j.cell.2005.11.044
Kaidi A, Weinert BT, Choudhary C, Jackson SP (2010) Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science 329(5997):1348–1353. https://doi.org/10.1126/science.1192049
Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V (2011) SIRT6 promotes DNA repair under stress by activating PARP1. Science 332(6036):1443–1446. https://doi.org/10.1126/science.1202723
Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R (2012) The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151(6):1185–1199. https://doi.org/10.1016/j.cell.2012.10.047
Dang CV, Semenza GL (1999) Oncogenic alterations of metabolism. Trends Biochem Sci 24(2):68–72
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64(11):3892–3899. https://doi.org/10.1158/0008-5472.can-03-2904
Dang CV (2013) MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med 3(8):a014217. https://doi.org/10.1101/cshperspect.a014217
Jin L, Alesi GN, Kang S (2016) Glutaminolysis as a target for cancer therapy. Oncogene 35(28):3619–3625. https://doi.org/10.1038/onc.2015.447
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458(7239):762–765. https://doi.org/10.1038/nature07823
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105(48):18782–18787. https://doi.org/10.1073/pnas.0810199105
Marbaniang C, Kma L (2018) Dysregulation of glucose metabolism by oncogenes and tumor suppressors in cancer cells. Asian Pac J Cancer Prev 19(9):2377–2390. https://doi.org/10.22034/apjcp.2018.19.9.2377
Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G (2009) Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ 16(1):87–93. https://doi.org/10.1038/cdd.2008.131
Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM (2006) DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126(1):121–134. https://doi.org/10.1016/j.cell.2006.05.034
Tasdemir E, Chiara Maiuri M, Morselli E, Criollo A, D’Amelio M, Djavaheri-Mergny M, Cecconi F, Tavernarakis N, Kroemer G (2008a) A dual role of p53 in the control of autophagy. Autophagy 4(6):810–814. https://doi.org/10.4161/auto.6486
Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F, Nannmark U, Samara C, Pinton P, Vicencio JM, Carnuccio R, Moll UM, Madeo F, Paterlini-Brechot P, Rizzuto R, Szabadkai G, Pierron G, Blomgren K, Tavernarakis N, Codogno P, Cecconi F, Kroemer G (2008b) Regulation of autophagy by cytoplasmic p53. Nat Cell Biol 10(6):676–687. https://doi.org/10.1038/ncb1730
Longley DB, Johnston PG (2005) Molecular mechanisms of drug resistance. J Pathol 205(2):275–292. https://doi.org/10.1002/path.1706
Zeller C, Dai W, Steele NL, Siddiq A, Walley AJ, Wilhelm-Benartzi CS, Rizzo S, van der Zee A, Plumb JA, Brown R (2012) Candidate DNA methylation drivers of acquired cisplatin resistance in ovarian cancer identified by methylome and expression profiling. Oncogene 31(42):4567–4576. https://doi.org/10.1038/onc.2011.611
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancer 6(3):1769–1792. https://doi.org/10.3390/cancers6031769
Maier P, Spier I, Laufs S, Veldwijk MR, Fruehauf S, Wenz F, Zeller WJ (2010) Chemoprotection of human hematopoietic stem cells by simultaneous lentiviral overexpression of multidrug resistance 1 and O(6)-methylguanine-DNA methyltransferase(P140K). Gene Ther 17(3):389–399. https://doi.org/10.1038/gt.2009.133
Valent P, Bonnet D, De Maria R, Lapidot T, Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi G, Huntly B, Herrmann H, Soulier J, Roesch A, Schuurhuis GJ, Wohrer S, Arock M, Zuber J, Cerny-Reiterer S, Johnsen HE, Andreeff M, Eaves C (2012) Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 12(11):767–775. https://doi.org/10.1038/nrc3368
Meacham CE, Morrison SJ (2013) Tumour heterogeneity and cancer cell plasticity. Nature 501:328. https://doi.org/10.1038/nature12624
Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, Kempski H, Moorman AV, Titley I, Swansbury J, Kearney L, Enver T, Greaves M (2011) Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469(7330):356–361. https://doi.org/10.1038/nature09650
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD, McMichael JF, Wallis JW, Lu C, Shen D, Harris CC, Dooling DJ, Fulton RS, Fulton LL, Chen K, Schmidt H, Kalicki-Veizer J, Magrini VJ, Cook L, McGrath SD, Vickery TL, Wendl MC, Heath S, Watson MA, Link DC, Tomasson MH, Shannon WD, Payton JE, Kulkarni S, Westervelt P, Walter MJ, Graubert TA, Mardis ER, Wilson RK, DiPersio JF (2012) Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481:506. https://doi.org/10.1038/nature10738
Zahreddine H, Borden KL (2013) Mechanisms and insights into drug resistance in cancer. Front Pharmacol 4:28. https://doi.org/10.3389/fphar.2013.00028
Aas T, Borresen AL, Geisler S, Smith-Sorensen B, Johnsen H, Varhaug JE, Akslen LA, Lonning PE (1996) Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 2(7):811–814
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW (1999) Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284(5411):156–159. https://doi.org/10.1126/science.284.5411.156
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4):395–402. https://doi.org/10.1016/j.ccr.2007.08.030
Dieras V, Vincent-Salomon A, Degeorges A, Beuzeboc P, Mignot L, de Cremoux P (2007) Trastuzumab (Herceptin) and breast cancer: mechanisms of resistance. Bull Cancer 94(3):259–266
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58. https://doi.org/10.1038/nrc706
Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S (2008) Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res 68(16):6533–6540. https://doi.org/10.1158/0008-5472.can-07-6642
Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, Green JE (2008) Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 68(15):6241–6250. https://doi.org/10.1158/0008-5472.can-07-6849
Papa S, Choy PM, Bubici C (2019) The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 38(13):2223–2240. https://doi.org/10.1038/s41388-018-0582-8
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/j.cell.2011.02.013
Maher JC, Wangpaichitr M, Savaraj N, Kurtoglu M, Lampidis TJ (2007) Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-D-glucose. Mol Cancer Ther 6(2):732–741. https://doi.org/10.1158/1535-7163.mct-06-0407
Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR (2007) 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 67(7):3364–3370. https://doi.org/10.1158/0008-5472.can-06-3717
Floridi A, Paggi MG, D’Atri S, De Martino C, Marcante ML, Silvestrini B, Caputo A (1981) Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res 41(11 Pt 1):4661–4666
Cao X, Fang L, Gibbs S, Huang Y, Dai Z, Wen P, Zheng X, Sadee W, Sun D (2007) Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol 59(4):495–505. https://doi.org/10.1007/s00280-006-0291-9
Chesney J (2006) 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care 9(5):535–539. https://doi.org/10.1097/01.mco.0000241661.15514.fb
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7(1):110–120. https://doi.org/10.1158/1535-7163.mct-07-0482
Wu MC, Arimura GK, Yunis AA (1978) Mechanism of sensitivity of cultured pancreatic carcinoma to asparaginase. Int J Cancer 22(6):728–733. https://doi.org/10.1002/ijc.2910220615
Griffiths M, Keast D, Patrick G, Crawford M, Palmer TN (1993) The role of glutamine and glucose analogues in metabolic inhibition of human myeloid leukaemia in vitro. Int J Biochem 25(12):1749–1755
Narta UK, Kanwar SS, Azmi W (2007) Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol 61(3):208–221. https://doi.org/10.1016/j.critrevonc.2006.07.009
Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A (2007) Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med 356(22):2282–2292. https://doi.org/10.1056/NEJMoa066596
Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15(4):254–266. https://doi.org/10.1016/j.semcancer.2005.04.005
Esslinger CS, Cybulski KA, Rhoderick JF (2005) Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem 13(4):1111–1118. https://doi.org/10.1016/j.bmc.2004.11.028
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534. https://doi.org/10.1016/j.cell.2008.11.044
Erickson JW, Cerione RA (2010) Glutaminase: a hot spot for regulation of cancer cell metabolism? Oncotarget 1(8):734–740. https://doi.org/10.18632/oncotarget.208
Qin JZ, Xin H, Nickoloff BJ (2010) Targeting glutamine metabolism sensitizes melanoma cells to TRAIL-induced death. Biochem Biophys Res Commun 398(1):146–152. https://doi.org/10.1016/j.bbrc.2010.06.057
Luo Z, Zang M, Guo W (2010) AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol 6(3):457–470. https://doi.org/10.2217/fon.09.174
Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB (2005) The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene 24(26):4165–4173. https://doi.org/10.1038/sj.onc.1208622
Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL (2007) Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110(1):313–322. https://doi.org/10.1182/blood-2006-10-050260
Nelson EC, Evans CP, Mack PC, Devere-White RW, Lara PN Jr (2007) Inhibition of Akt pathways in the treatment of prostate cancer. Prostate Cancer Prostatic Dis 10(4):331–339. https://doi.org/10.1038/sj.pcan.4500974
Kong D, Yamori T (2008) Phosphatidylinositol 3-kinase inhibitors: promising drug candidates for cancer therapy. Cancer Sci 99(9):1734–1740. https://doi.org/10.1111/j.1349-7006.2008.00891.x
Guertin DA, Sabatini DM (2009) The pharmacology of mTOR inhibition. Sci Signal 2(67):pe24. https://doi.org/10.1126/scisignal.267pe24
Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 94(13):6658–6663. https://doi.org/10.1073/pnas.94.13.6658
Hagland H, Nikolaisen J, Hodneland LI, Gjertsen BT, Bruserud O, Tronstad KJ (2007) Targeting mitochondria in the treatment of human cancer: a coordinated attack against cancer cell energy metabolism and signalling. Expert Opin Ther Targets 11(8):1055–1069. https://doi.org/10.1517/14728222.11.8.1055
Turton JL, Raab R, Rooney KB (2018) Low-carbohydrate diets for type 1 diabetes mellitus: a systematic review. PLoS One 13(3):e0194987. https://doi.org/10.1371/journal.pone.0194987
Buyken AE, Toeller M, Heitkamp G, Irsigler K, Holler C, Santeusanio F, Stehle P, Fuller JH (2000) Carbohydrate sources and glycaemic control in Type 1 diabetes mellitus. EURODIAB IDDM Complications Study Group. Diabet Med 17(5):351–359
Cheung NW, Conn JJ, d’Emden MC, Gunton JE, Jenkins AJ, Ross GP, Sinha AK, Andrikopoulos S, Colagiuri S, Twigg SM (2009) Position statement of the Australian Diabetes Society: individualisation of glycated haemoglobin targets for adults with diabetes mellitus. Med J Aust 191(6):339–344
Hall KD, Chung ST (2018) Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care 21(4):308–312. https://doi.org/10.1097/mco.0000000000000470
Taylor R, Barnes AC (2018) Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia 61(2):273–283. https://doi.org/10.1007/s00125-017-4504-z
Mansoor N, Vinknes KJ, Veierod MB, Retterstol K (2016) Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr 115(3):466–479. https://doi.org/10.1017/s0007114515004699
Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD (2015) The role of protein in weight loss and maintenance. Am J Clin Nutr 101(6):1320s–1329s. https://doi.org/10.3945/ajcn.114.084038
Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, Tragante V, van Iperen EP, Sivapalaratnam S, Shah S, Elbers CC, Shah T, Engmann J, Giambartolomei C, White J, Zabaneh D, Sofat R, McLachlan S, Doevendans PA, Balmforth AJ, Hall AS, North KE, Almoguera B, Hoogeveen RC, Cushman M, Fornage M, Patel SR, Redline S, Siscovick DS, Tsai MY, Karczewski KJ, Hofker MH, Verschuren WM, Bots ML, van der Schouw YT, Melander O, Dominiczak AF, Morris R, Ben-Shlomo Y, Price J, Kumari M, Baumert J, Peters A, Thorand B, Koenig W, Gaunt TR, Humphries SE, Clarke R, Watkins H, Farrall M, Wilson JG, Rich SS, de Bakker PI, Lange LA, Davey Smith G, Reiner AP, Talmud PJ, Kivimaki M, Lawlor DA, Dudbridge F, Samani NJ, Keating BJ, Hingorani AD, Casas JP (2015) Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 36(9):539–550. https://doi.org/10.1093/eurheartj/eht571
Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM (2015) Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr 35:517–543. https://doi.org/10.1146/annurev-nutr-071714-034449
Lennerz BS, Barton A, Bernstein RK, Dikeman RD, Diulus C, Hallberg S, Rhodes ET, Ebbeling CB, Westman EC, Yancy WS (2018) Management of type 1 diabetes with a very low–carbohydrate diet. Pediatrics 141(6):e20173349
Ma S, Suzuki K (2019) Keto-adaptation and endurance exercise capacity, fatigue recovery, and exercise-induced muscle and organ damage prevention: a narrative review. Sports 7(2):40
Gasior M, French A, Joy MT, Tang RS, Hartman AL, Rogawski MA (2007) The anticonvulsant activity of acetone, the major ketone body in the ketogenic diet, is not dependent on its metabolites acetol, 1,2-propanediol, methylglyoxal, or pyruvic acid. Epilepsia 48(4):793–800
McNally MA, Hartman AL (2012) Ketone bodies in epilepsy. J Neurochem 121(1):28–35
Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA (2017) A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26(3):539–546
Chang C-K, Borer K, Lin P-J (2017) Low-carbohydrate-high-fat diet: can it help exercise performance? J Hum Kinet 56(1):81–92
Weber DD, Aminazdeh-Gohari S, Kofler B (2018) Ketogenic diet in cancer therapy. Aging 10(2):164
Morscher RJ, Aminzadeh-Gohari S, Feichtinger RG, Mayr JA, Lang R, Neureiter D, Sperl W, Kofler B (2015) Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model. PLoS One 10(6):e0129802
Hrynevich SV, Waseem TV, Hébert A, Pellerin L, Fedorovich SV (2016) β-Hydroxybutyrate supports synaptic vesicle cycling but reduces endocytosis and exocytosis in rat brain synaptosomes. Neurochem Int 93:73–81
Gano LB, Patel M, Rho JM (2014) Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res 55(11):2211–2228
Ghosh S, Castillo E, Frias ES, Swanson RA (2018) Bioenergetic regulation of microglia. Glia 66(6):1200–1212
Yang X, Cheng B (2010) Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci 42(2):145–153
Stephens KE, Miaskowski CA, Levine JD, Pullinger CR, Aouizerat BE (2013) Epigenetic regulation and measurement of epigenetic changes. Biol Res Nurs 15(4):373–381
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339(6116):211–214
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173(7):1728–1741
Pekun TG, Lemeshchenko VV, Lyskova TI, Waseem TV, Fedorovich SV (2013) Influence of intra-and extracellular acidification on free radical formation and mitochondria membrane potential in rat brain synaptosomes. J Mol Neurosci 49(1):211–222
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ain, Nu., Gull, H. (2020). Metabolic Changes and Their Characterization. In: Masood, N., Shakil Malik, S. (eds) 'Essentials of Cancer Genomic, Computational Approaches and Precision Medicine. Springer, Singapore. https://doi.org/10.1007/978-981-15-1067-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-1067-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1066-3
Online ISBN: 978-981-15-1067-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)