Abstract
The striatum is a key neural substrate that controls selection of reward-seeking and aversive behaviors (Bromberg-Martin et al., Neuron, 2010; Graybiel, Annu Rev Neurosci, 2008). Striatal transmission is mediated by two distinct neuronal subpopulations consisting of D1 and D2 receptor-expressing medium spiny neurons (dMSNs, iMSNs) and is regulated by protein kinase A (PKA) and extracellular signal-regulated kinase (ERK) cascades (Luo et al., J. Neurosci, 2011). However, the in vivo roles and signaling mechanisms of dMSNs and iMSNs remain largely elusive, because of the lack of techniques to monitor such kinase activities in behaving animals. Here we report that the activities of PKA and ERK are coordinately and reciprocally regulated in dMSNs and iMSNs of the dorsal striatum by rewarding and aversive stimuli. This finding was made by developing a novel in vivo method in which Förster resonance energy transfer (FRET) biosensors of PKA and ERK were specifically expressed in either dMSNs (D1-PKA and D1-ERK) or iMSNs (D2-PKA and D2-ERK) (Kamioka et al., Cell Struct Funct, 2012; Gong et al., J Neurosci, 2007; Valjent et al., Trends Neurosci, 2009), with fluorescence changes optically recorded by micro-endoscopy in freely moving mice. Cocaine administration rapidly and continuously activated both D1-PKA and D1-ERK but markedly suppressed both D2-PKA and D2-ERK. Conversely, prominent activation of D2-PKA and D2-ERK together with strong suppression of D1-PKA and D1-ERK was evoked in response to electric foot shocks. Importantly, D1-PKA and D1-ERK were activated during the mating reaction of male mice; but D1-PKA was promptly inactivated upon ejaculation. In contrast, D2-PKA and D2-ERK were elevated when male mice became indifferent to mating. Manipulation of cAMP levels by DREADDs successfully induced PKA response of either dMSNs or iMSNs and concomitantly affected mating behaviors, indicating its causality. Thus, a dynamic regulatory shift of PKA and ERK between dMSNs and iMSNs underlies the rapid selection of reward-directed and aversive behaviors.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keyword
Cell-specific, time-lapsed changes in activities of dMSNs and iMSNs were examined by biosensors that allowed monitoring the active and inactive forms of PKA and ERK (Kamioka et al., Cell Struct. Funct. 2012). Four lines of transgenic mice were generated by crossing two lines of biosensor-expressing transgenic mice (floxed AKAR3EV for PKA and floxed EKAREV for ERK) with D1-Cre and D2-Cre BAC transgenic mice (Goto et al., PNAS, 2015, Fig. 8.1a). The D1-PKA and D1-ERK mice and the D2-PKA and D2-ERK mice exclusively expressed the respective FRET biosensors in the neural pathways of dMSNs and iMSNs, respectively. FRET imaging of the striatum of freely moving mice was achieved by developing fiber bundle-based micro-endoscope techniques (Fig. 8.1b). The pencil like-shaped tip of the optical fiber bundle was implanted into the mouse brain, and the flat end of the bundle was scanned with a confocal laser scanning microscope equipped with a 445-nm laser for excitation and a pair of band-path filters, 483 ± 16 nm for CFP and 542 ± 13 nm for FRET. Fluorescence excitation of the FRET biosensors was exclusively detected in the striatum of the transgenic mice. Upon quantitative analysis, in vivo administration of SP-8-Br-cAMPs (cAMP analog) through the cannula attached close to the endoscope caused a progressive increase in FRET responses (changes in FRET/CFP ratio) in both D1-PKA and D2-PKA mice (Fig. 8.1c), but not in PKA-neg mice. Conversely, the MEK inhibitor PD184352 gradually decreased FRET responses in both D1-ERK and D2-ERK mice (Fig. 8.1c). The FRET micro-endoscopy thus allowed us to monitor dynamic changes in activities of PKA and ERK specific for dMSNs and iMSNs.
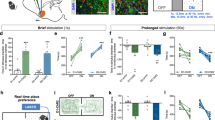
Live monitoring of PKA and ERK activities in dMSNs and iMSNs of transgenic mice expressing FRET biosensors
(a) Construct for Cre-dependent expression of FRET biosensors
(b) Schema of the micro-endoscope system. Changes in FRET responses were monitored to measure the activity of PKA or ERK
(c) Local application of SP-8-Br-cAMP (2 mM) and PD184352 (10 μM) into the dorsal striatum of anesthetized transgenic mice (indicated by black bars) respectively increased FRET responses of D1-PKA and D2-PKA and decreased those of D1-ERK and D2-ERK
Exposure to cocaine causes a massive increase in the dopamine (DA) level in the striatum and results in activation of PKA and ERK in this brain region (Valjent et al., J. Neurosci, 2000; Bertran-Gonzales et al., J. Neurosci, 2008). Upon FRET micro-endoscopic analysis, administration of cocaine (25 mg/kg, i.p.), but not that of saline, rapidly and continuously increased the activities of both D1-PKA and D1-ERK with a concomitant increase in locomotor activity (Fig. 8.2a). This increase was in marked contrast to the response of iMSNs, in which the activities of both D2-PKA and D2-ERK were gradually decreased by the cocaine administration (Fig. 8.2a). Importantly, saline injection appreciably activated both D2-PKA and D2-ERK (Fig. 8.2a). Aversive stimuli transiently suppress tonic firings of most of DA neurons in the ventral tegmental area and disinhibit iMSNs via inactivation of Gi-coupled inhibitory D2 receptors (Gerfen and Surmeier, Annu. Rev. Neurosci, 2011; Ungless, Science, 2004). Because saline injection supposedly serves as an aversive stimulus, we more directly addressed how a strong aversive stimulus, i.e., an electric foot shock, would affect PKA and ERK in dMSNs and iMSNs. Both D2-PKA and D2-ERK were markedly activated in response to an electric foot shock (2 mA, 40 Hz for 2 sec) (Fig. 8.2b). Conversely, the activities of D1-PKA and D1-ERK were notably decreased in response to the electric shock (Fig. 8.2b). Thus, the activities of PKA and ERK are reciprocally controlled not only by rewarding and aversive stimuli but also between dMSNs and iMSNs. We further investigated involvement of D2-PKA and D2-ERK in the induction of aversive learning by pairing electric shocks with the sound of a bell (Fig. 8.2b). After habituation with repeated exposure to a bell sound, an additional bell sound never influenced the activities of PKA and ERK in either dMSNs or iMSNs and thus served as neutral information. Notably, both PKA and ERK were activated and inactivated in iMSNs and dMSNs, respectively, by the conditioned bell sound without electric shocks. Thus, the cell-specific regulation of PKA and ERK underlies the induction of both the acute aversive reaction and aversive learning behavior.
Reciprocal regulation of PKA and ERK activities between dMSNs and iMSNs in response to cocaine administration and electric foot shocks
(a) PKA and ERK activities (upper traces) with SEM (vertical lines) and locomotor activities (lower traces) in response to cocaine or saline injection
(b) PKA and ERK activities in response to electric foot shocks (Shock), bell sound without unconditioned stimulus (NCS), and bell sound after conditioning with electric shocks (CS)
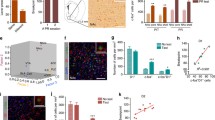
Naturally occurring rewarding stimuli such as food, drinking, and mating enhance DA release in the striatum and induce motivational reward-seeking behavior (Damsma et al., Behav. Neurosci, 1992; Salamone and Correa, Neuron, 2012). The role of striatal PKA and ERK in naturally occurring rewarding behavior was addressed by measuring the activities of PKA and ERK during the mating behavior of a male mouse after inclusion of a female mouse in the same chamber. The male mice showed a wide variety of mating reactions, including frequent sniffing, grooming, and mounting (Park 2011). We counted the percentages of time exhibiting mating behaviors (sniffing, grooming, mounting) every minutes during a 30-min period after inclusion of the female mouse (%PMR), and used it as an index to estimate the motivational strength for the male mice to the presented female mouse. When overall 30 min period (interaction with female) was averaged, a significant positive correlation was noted between the extent of the D1-PKA and D1-ERK activation and the percentage of positive mating reactions, and this correlation was inverted in the responses of D2-PKA and D2-ERK (Fig. 8.3a). When mating behaviors were arbitrarily divided into two groups exhibiting either frequent or infrequent mating reactions by values of 20% PMR (Fig. 8.3b), the D1-PKA and D1-ERK activities significantly increased in frequently interacting mice, whereas the D2-PKA and D2-ERK activities were increased in the infrequently interacting ones (Fig. 8.3b). We noticed that the observed activity changes in frequently and infrequently interacting animals depended on whether these mice became successively motivated or indifferent to a female mouse during mating behaviors. Thus, the PKA and ERK activities of individual mice were pursued during the shift between the motivational phase and indifferent phase of mating behavior (defined as more than 50% PMR increase and decrease within 1–4 min, respectively) (Fig. 8.3c). This analysis revealed that the activities of D1-PKA and D1-ERK were elevated during the motivational phase of the mating reaction. In contrast, the activities of D2-PKA and D2-ERK were increased when a male mouse became indifferent to or escaped from a female mouse. Thus, the activities of both PKA and ERK (but more PKA) rapidly changed during mating behavior, and this rapid change reflected the shift between motivational and indifferent phases of the mating reaction. The above results suggest that activation of PKA and ERK in dMSNs and iMSNs underlies the induction and suppression of mating reactions, respectively. To substantiate this possibility, the ejaculation of a male mouse was facilitated by pairing the male mouse with a hormonally-primed female mouse (Ogawa et al., Endocrinology, 1998) and the activities of D1-PKA and D1-ERK were measured before and after ejaculation (Fig. 8.3e, f). The D1-PKA activity elevated during the mating reaction was rapidly reduced to lower levels within 4 min after ejaculation, whereas the D1-ERK activity continued to be elevated even after ejaculation, suggesting that PKA in the dMSNs was more relevantly associated with the rapid shift in the mating reaction.
PKA and ERK activities of dMSNs and iMSNs during sexual interaction
(a) A male mouse was exposed to an unfamiliar female mouse for 30 min and analyzed by the FRET micro-endoscopy. Percentages of PMR were measured, and average changes in activities of PKA and ERK of dMSNs and iMSNs during the 30-min period were plotted against % PMR
Fig. 8.3 (continued) (b) Changes in activities of PKA and ERK (upper traces) and locomotor activities (lower traces) of frequently or infrequently interacting male mice
(c, d) Up- and down-regulation of PKA and ERK activities at the active and indifferent phases of the mating reaction(∗p < 0.05, Mann-Whitney’s U test). The numbers in parentheses indicate the sample numbers from 6 to 9 animals
(e), (left) Examples of changes in the PKA and ERK activities in dMSNs after ejaculation. Ejaculation is marked with the arrow in the D1-PKA (red) and D1-ERK (black) mouse
(right) Rapid inactivation of PKA in the D1-PKA mice after ejaculation (∗p < 0.05, n = 5, Wilcoxon signed-rank test)
To further address the causality of PKA activity in mating behavior, we artificially activated Gi or Gs by DREADDs (Designer Receptors exclusively Activated by a Designer Drug, Rogan & Roth, Pharmacological reviews, 2011) which were induced by adeno-associate virus in a Cre-dependent manner. CNO was injected 10 or 20 min after the female entry. Around 10 min after CNO injection, PKA activity was either decreased in AAV-hM4Di-mCherry injected mice, or increased in AAV-hDs-mCherry injected ones (Goto et al., PNAS, 2015). Concomitant to the increase or decrease in D1-PKA, male mouse exhibited increase or decrease in %PMR, respectively. Conversely, increase or decrease in D2-PKA either induced either decrease or increase in %PMR, respectively. These results indicate that the causal relationship between PKA activity of dMSNs and iMSNs in the dorsal striatum and the mating reactions in male mice.
Figure 8.4 summarizes differences in the amplitudes of the PKA and ERK responses in MSNs along different behavioral conditions. This summary explicitly demonstrates that PKA and ERK are coordinately stimulated or inhibited in both dMSNs and iMSN but oppositely regulated between these two cell types under different conditions. Animal behaviors can thus be orderly aligned from rewarding to aversive behaviors by taking into account the extents of activation and inactivation of PKA and ERK in individual behaviors. Intriguingly, highly interacting male mice with female mice exhibited higher PKA and ERK activities in dMSNs than those of cocaine-treated mice, suggesting that sexual behavior is highly emotional and motivational for male mice. Importantly, the motivational and indifferent phases of mating reactions are tightly associated with the rapid shift in the activities of PKA and ERK in both dMSNs and iMSNs. Phosphorylation/dephosphorylation of PKA and ERK in these two types of MSNs could thus be a potential mechanism that controls this rapid shift between rewarding and aversive behaviors. Notably, the bell sound after conditioning with electric shocks induced profound effects on both dMSNs and iMSNs, indicating that robust and strong adaptive alterations in the striatal circuit are involved in the aversive learning behavior. Furthermore, saline injection, spontaneous locomotion, and indifference of male mice toward female mice caused up-regulation of the activities of PKA and ERK in iMSNs. This finding is consistent with the predominant expression of high-affinity (nM order) D2 receptors in iMSNs, which are capable of sensing subtle changes in synaptic DA concentrations in the striatum (Gerfen & Surmeier, Annu. Rev. Neurosci, 2011). The iMSN transmission would thus greatly contribute to the innate tendency of animals to be more concerned about and to rapidly avoid uncomfortable environments and predators. Conversely, when animals encounter rewarding stimuli such as sexual interaction, these stimuli increase DA levels in the striatum and stimulate low-affinity (μM order) D1 receptors in dMSNs. Thus, the D1 and D2 receptors serve as key determinants to distinctly sense changes in synaptic DA transmission in a pathway-specific manner and to induce reward-directed and aversive behaviors via common PKA and ERK signaling cascades (Nakanishi et al., Neuroscience, 2014).
Summary of PKA and ERK activities under rewarding and aversive/impassive conditions
Values of the PKA and ERK activities were calculated by averaging FRET responses during 5 min at the peak response under the different conditions. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, as compared with the non-conditioned bell sound (Mann-Whitney’s U test). Reward-seeking and aversive/impassive animal behaviors can be orderly aligned according to the extents of activation and inactivation of PKA and ERK
We also applied these techniques to measure the temporal dynamics of PKA response in the formation of aversive memory in the core part of nucleus accumbens (NAc). We found that PKA activities of iMSNs at NAc occurred not instantaneously after footshock but in a delayed and progressive manner (Yamaguchi et al., PNAS, 2015). We believe that the above methodologies allow us to study regulatory mechanisms of neural circuits involved in a wide range of animal behaviors.
References
Bertran-Gonzalez J et al (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685
Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834
Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC (1992) Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci 106:181–191
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466
Gong S et al (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27:9817–9823
Goto A, Nakahara I, Yamaguchi T, Kamioka Y, Sumiyama K, Matsuda M, Nakanishi S, Funabiki K (2015) Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci U S A 112(21):6718–6723
Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31:359–387
Kamioka Y et al (2012) Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct Funct 37:65–73
Luo Z, Volkow ND, Heintz N, Pan Y, Du C (2011) Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: in vivo optical microprobe [Ca2+]i imaging. J Neurosci 31:13180–13190
Nakanishi S, Hikida T, Yawata S (2014) Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience 282:49–59
Ogawa S et al (1998) Modifications of testosterone-dependent behaviors by estrogen receptor-α gene disruption in male mice 1. Endocrinology 139:5058–5069
Park JH (2011) Assessment of male sexual behavior in mice. In: Mood and anxiety related phenotypes in mice, vol 63. Humana Press, New York, pp 357–373
Rogan SC, Roth BL (2011) Remote control of neuronal signaling. Pharmacol Rev 63(2):291–315
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485
Ungless MA (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2042
Valjent E et al (2000) Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20:8701–8709
Valjent E, Bertran-Gonzalez J, Hervé D, Fisone G, Girault J-A (2009) Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 32:538–547
Yamaguchi T, Goto A, Nakahara I, Yawata S, Hikida T, Matsuda M, Funabiki K, Nakanishi S (2015) Role of PKA signaling in D2 receptor-expressing neurons in the core of the nucleus accumbens in aversive learning. Proc Natl Acad Sci U S A 112(36):11383–11388
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Supplementary Electronic Material (S)
(MP4 458030 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Funabiki, K. (2020). Circuit-Dependent Striatal PKA and ERK Signaling Underlying Action Selection. In: Toyama, Y., Miyawaki, A., Nakamura, M., Jinzaki, M. (eds) Make Life Visible. Springer, Singapore. https://doi.org/10.1007/978-981-13-7908-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-7908-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7907-9
Online ISBN: 978-981-13-7908-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)