Abstract
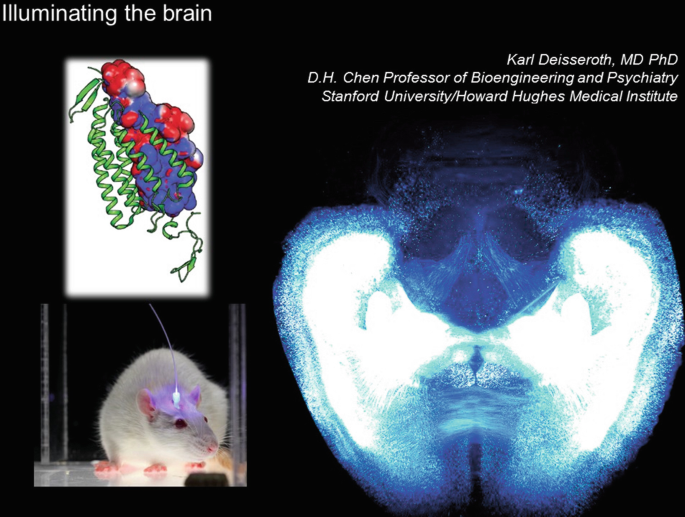
The brain, like other living systems, tolerates light, which can be delivered flexibly over a range of wavelengths and irradiance values. The fact that most cells do not respond to light provides an enormous signal-to-noise advantage—if any light sensitivity can be conferred. Our efforts on using light to illuminate brain structure and function have taken us from the basic science investigation of microbial protein crystal structures, to optical probing of brain structure and function using hydrogel-tissue chemistry and optogenetics.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
-
1.
In 1972, Karl Hartmann enumerated the reasons that light is a uniquely suited tool for probing living systems. Though coming from the perspective of spectroscopy, his perceptive list holds true even for systems as complex as the adult mammalian brain.
-
2.
The brain, like other living systems, tolerates light, which can be delivered flexibly over a range of wavelengths and irradiance values. Crucially, working with the instantaneous speed and inertia-less nature of light, at least as far as biological spatial and temporal scales are concerned, brings immense value for using light as a control and readout tool in biology. And the fact that most cells do not respond to light provides an enormous signal-to-noise advantage—if any light sensitivity can be conferred.
-
3.
Our efforts on using light to illuminate brain structure and function have taken us from the basic science investigation of microbial protein crystal structures, to optical probing of brainwide wiring and gene expression patterns, to optogenetics (development and application of methods for using light delivered via fiberoptics to control single cells or kinds of cells in the brains of behaving mammals).
-
4.
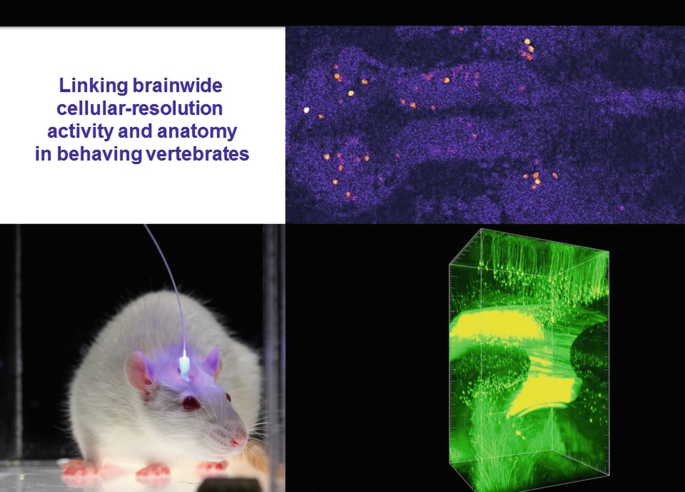
The biggest challenge facing us now is integrating the following datastreams at cellular resolution across the brain: (1) natural activity during behavior (with fluorescence Ca2+ imaging), (2) causal activity during behavior (with optogenetics), and (3) local/global wiring with gene expression (via hydrogel-tissue chemistry methods such as CLARITY).
-
5.
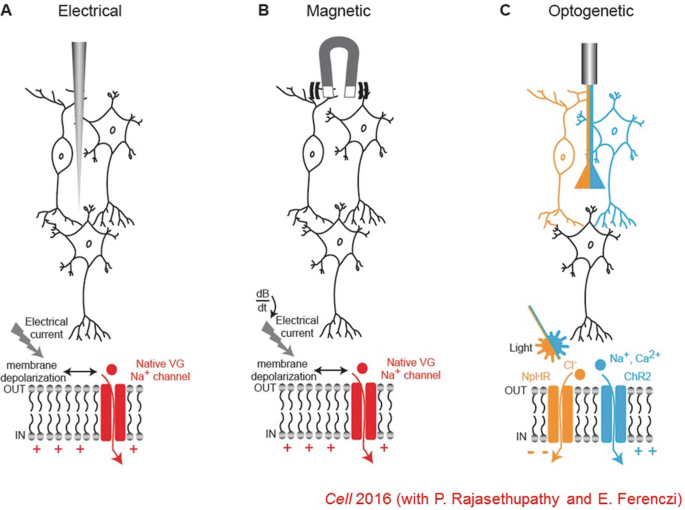
Electrical and magnetic interventions, while fast, cannot readily provide cell-type specificity—since all neurons respond to these signals. Optogenetics is a way of providing cellular resolution with cell-type specificity by expressing a light transducer in targeted cells—which allows use of a vastly more tractable signal since virtually no neurons respond to light under normal conditions.
-
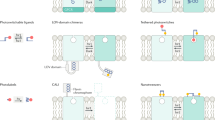
6.
This method works well if the light transducer is also a cell-activity effector—thus all-in-one microbial opsin proteins provided the optimal solution, since their single polypeptide chain (bound to a naturally-present chromophore) can both absorb a photon and generate current. The channelrhodopsin is an interesting member of this family since it can move many ions across the membrane, in response to light by opening its channel pore. But while structural information on non-channel (pump) members of this protein family has been available for decades, the channelrhodopsins remained mysterious until events of the last few years.
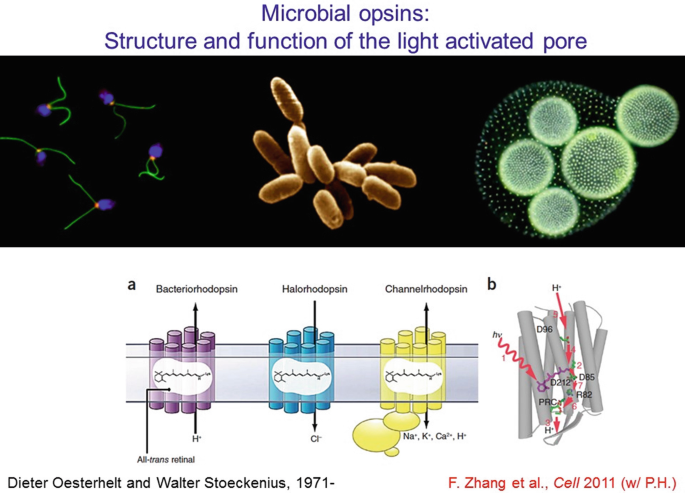
-
7.
Detailed protein biophysics and chemistry, involving crystal-structure determination, molecular dynamics modeling, and structure/model-guided redesign, have given rise to fundamentally distinct forms of photon-spike coupling. In 2010, ultrafast coupling was created that allowed control of spiking at 200 Hz or more. In 2011, control of spiking with red light was implemented. In 2008, bistable cellular control of excitation was implemented, in which single pulses of light cause stable excitation (which can last many minutes until reversed with a pulse of redshifted light). And in 2014 bistable cellular inhibition was implemented, via creation of anion-conducting channelrhodopsins (through pore redesign for chloride flux followed by porting the bistability mutations onto the anion-conducting backbone). Each of these variants was designed and implemented using atomic-structure-level modeling and understanding.
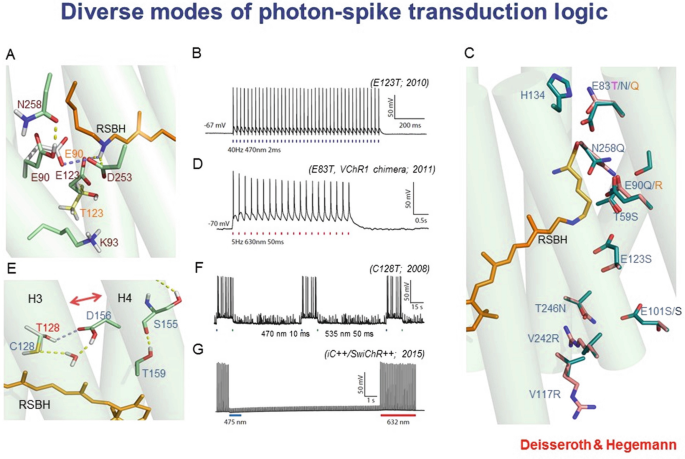
-
8.
The ChETA mutations of channelrhodopsins allowed the first ultrafast spiking over hundreds of Hz in fast-spiking neurons, in vitro and in vivo, and also corrected some fidelity breakdown issues that occur even at low spike rates.
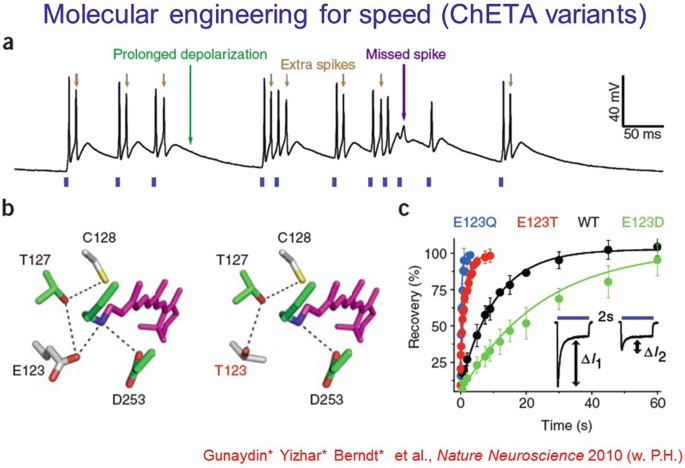
-
9.
The SFO mutants represent the bistable type described above. The normally-fast (10 ms) deactivation after light-off of wild-type channelrhodospins can be extended to 30 min or more with mutations at the “DC bridge” (C128 and D156). Orders-of-magnitude greater operational light sensitivity in expressing cells is also achieved.
-
10.
Resolution of the crystal structure finally demonstrated the nature of the pore, residing within each 7-TM monomer and not at the dimer interface as had been suggested by a low-resolution cryo-EM paper.
-
11.
Obtaining the pore structure allow for redesign for new function. It was noted that a preponderance of electronegativity characterized the inner lining of the pore for these naturally cation-selective channels, suggesting a strategy to test a hypothesis for selectivity and create a new tool, by replacing these residues to create electropositivity and perhaps anion flux, which would be of great value for inhibitory optogenetics.
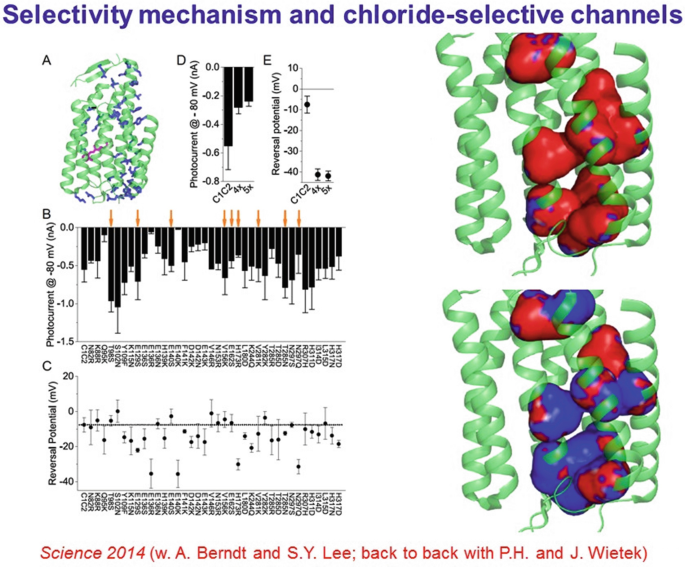
-
12.
Surprisingly, this experiment was successful, and inhibitory channels became available which could shut down spiking in response to blue light, rather than drive spiking.
-
13.
Bistable inhibition (a long-sought property for biologists doing optogenetics) then was enabled by porting the bistability mutations onto this new backbone.
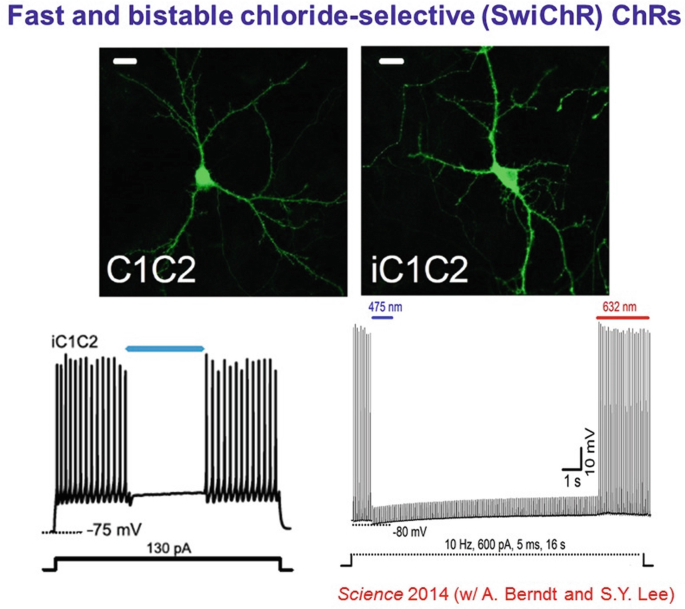
-
14.
A year later, naturally-occurring light-activated chloride channels were discovered in the Guillardia theta algae. Validating yet further the structure-guided mutagenesis that had created the initial anion-conducting channelrhodopsins, the internal pore electrostatics of the naturally-occurring channels were predicted to be largely positive, similar to the redsigned channelrhodopsins.
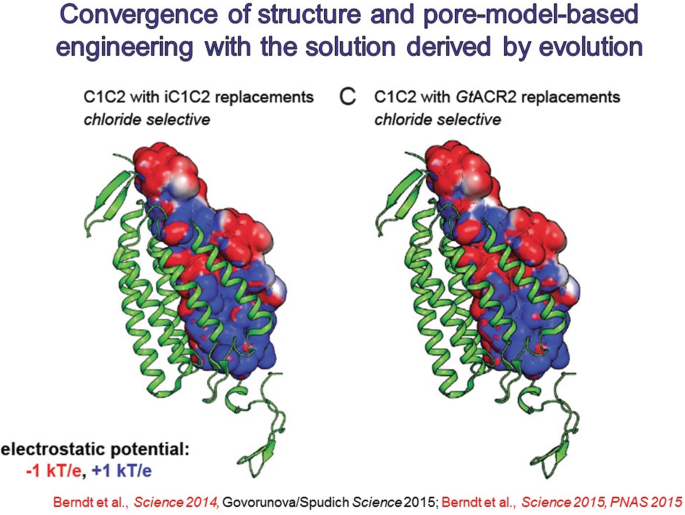
-
15.
Color shifting is more challenging, but was ultimately achieved beginning in 2008 when a naturally-occurring redshifted variant called VChR1 was identified from the multicellular green alga Volvox carteri.
-
16.
With additional mutagenesis of VChR1 (including creating a chimera with ChR1, and improving cellular trafficking) by 2011 red light-driven spiking was enabled with C1V1.
-
17.
The greatest value of C1V1 and subsequent other red-driven opsins has been in integration with blue light-driven Ca2+ imaging at cellular resolution—resulting in all-optical experimentation. This can be done at the cellular level with single-cell-directed light guidance, or at the population level with fiber photometry. Either way, naturally-occurring activity as seen during behavior within defined cells or cell types can now be mimicked (or adjusted) in terms of timing and magnitude—allowing rigorous assessment of causal significance of naturally-occurring signals.
-
18.
Regarding applications to such questions, optogenetics has been applied to a very broad range of natural and disease-relevant behaviors. A well-studied example is anxiety; the cells and projections across the brain that recruit the various features of anxiety, such as risk-avoidance, altered respiratory rate, and negative subjective valence, can all be now assigned to contributory specific projections defined by origin and target, with specificity arising from optogenetics. Brain states could be shown (with optogenetics) to be assembled from these features defined by brainwide projections.
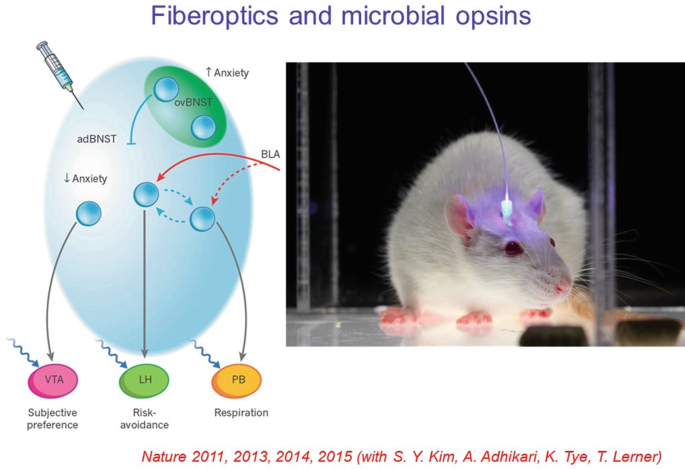
-
19.
Another example of both basic and disease-relevant interest is the behavioral state transition from active-coping to passive-coping in response to a challenge. This is a naturally adaptive transition, but also in extreme forms can be problematic in human clinical states such as depression. This process has been studied in rats in the forced swim test (FST), and optogenetics has been used to test causal significance of cells and projections across the brain in this very important (and often adaptive) state transition.
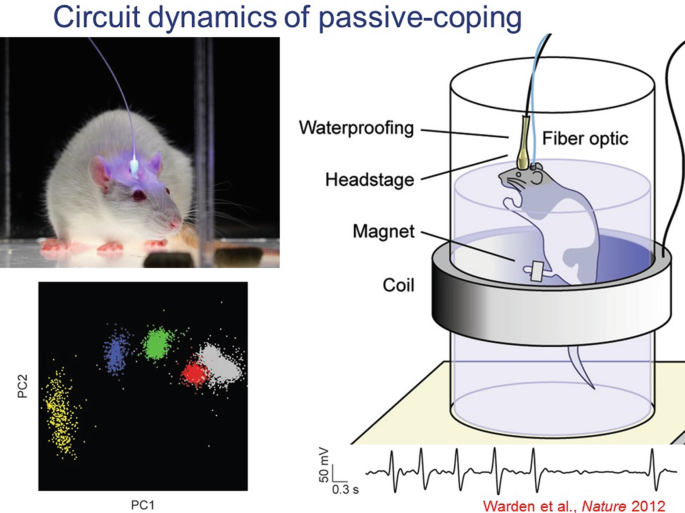
-
20.
Many individual regions across the brain had been implicated, but specific causal projections and pathways involved were not known.
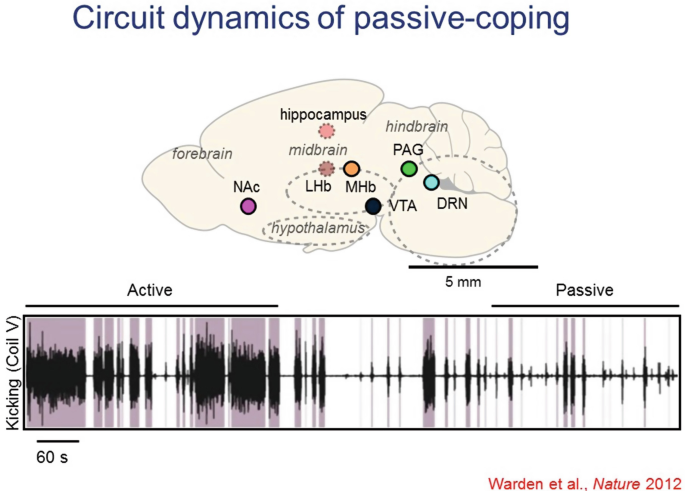
-
21.
Driving the projection from the medial prefrontal cortex (mPFC) to the lateral habenula favored the passive-coping state, quantified as reduced kicking and swimming in the FST. These effects were not seen in non-challenging situations (no psychomotor changes in the open-field locomotion test for example) and were not seen with nonspecific optogenetic drive of all mPFC neurons, nor of mPFC projections to basolateral amygdala.
-
22.
In contrast, driving the projection from the mPFC to the dorsal raphe nucleus favored the active-coping state (again with no effect in the open field). However, these point-by point investigations may miss unanticipated loci of control, and do not represent brainwide joint statistics. We therefore are seeking to develop brainwide cellular-resolution assays.
-
23.
Zebrafish provide a valuable approach for initially exploring this avenue, particularly since we have developed truly brainwide cellular-resolution Ca2+ imaging assays.
-
24.
This two-photon imaging approach provides the bona fide cellular resolution needed for linking cellular activity to cellular identity. In headfixed zebrafish (with tail free to assess direction and vigor of exertion) the brainwide cellular activity patterns relevant to behavior can be assessed without regional bias or preconception.
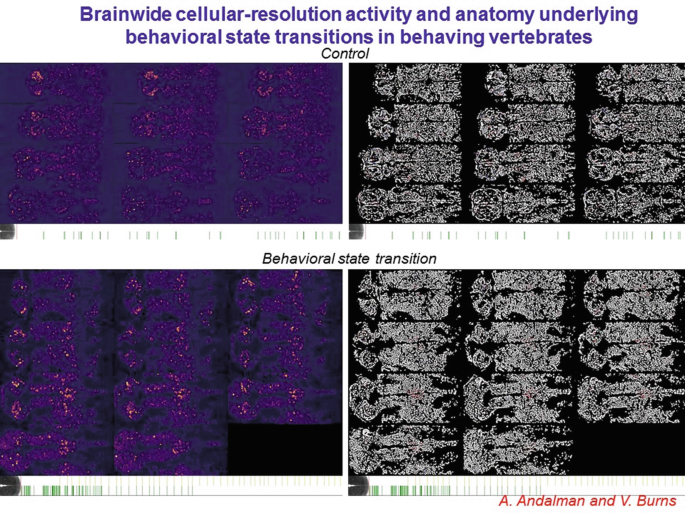
-
25.
Moreover, with technology recently developed in our group, those same cells can be then further interrogated for detailed molecular phenotyping, including gene expression and neurotransmitter identity. This requires computational approaches to morph the during-life activity-dataset to the post-life molecular-phenotype-datasets, which do not readily align without the computational warping approach (termed MultiMAP and recently published in Cell 2017 from Matt Lovett-Barron and co-workers in my lab).
-
26.
This method in the end has finally enabled our linkage of brainwide anatomical identity/molecular phenotype, to cellular-resolution activity during behavior, an approach that will open the door to a very wide array of investigations in neuroscience (both basic and preclinical). The final piece of the puzzle is local and global wiring of these same cells, now addressable with hydrogel-tissue chemistry methods such as CLARITY.
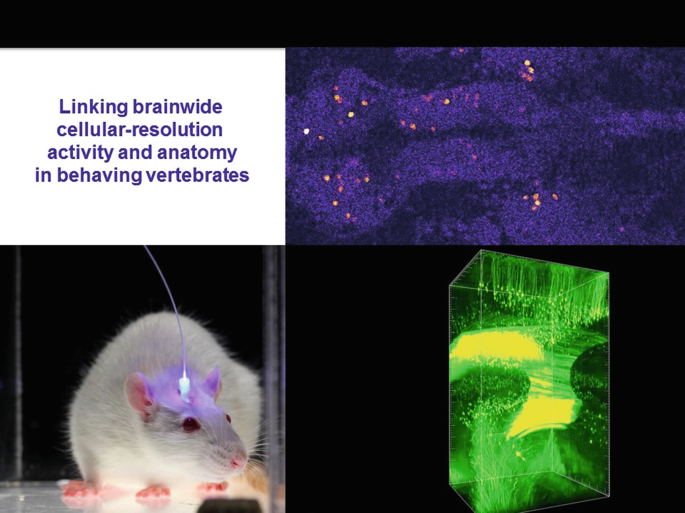
-
27.
With hydrogel-tissue chemistry, specific classes of native biomolecules in tissue are immobilized or covalently anchored (for example through individualized interface molecules to gel monomer molecules) and precisely-timed polymerization causing tissue-gel hybrid formation is triggered within all the cells across the tissue in an ordered and controlled process to create an optically and chemically accessible biomolecular matrix. Indeed, when the biomolecules of interest are thereby transferred to the polymer lattice, a robust new composite hydrogel-tissue material results, which becomes the substrate for future chemical and optical interrogation that can be probed and manipulated in new ways. Moderate tissue expansion occurs as was shown in 2013 and 2014, but this can be reversed if needed with a refractive index matching solution.
-
28.
Hydrogel-tissue chemistry is particularly well suited for nucleic acid interrogation, especially with the EDC CLARITY method—important for rich typology labeling.
-
29.
This approach also opens the door to immediate-early gene tracking at cellular resolution for genes such as Arc, as well as noncoding RNAs such as microRNAs.
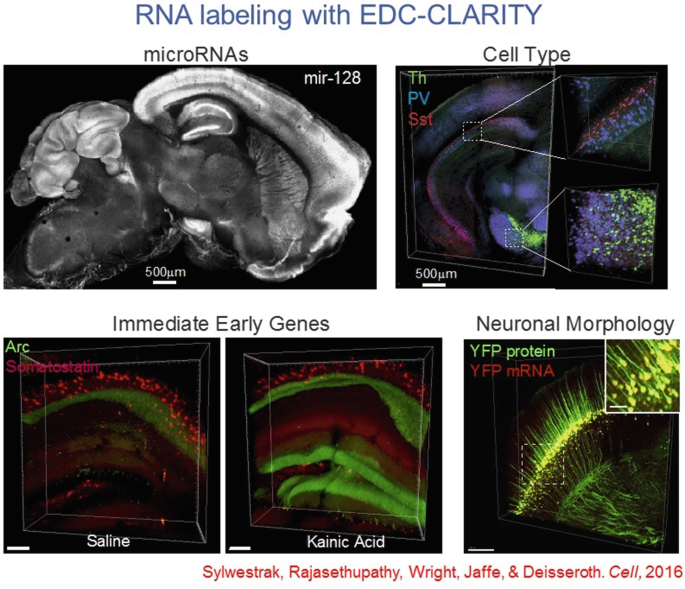
-
30.
A bottleneck initially was imaging speed; high-resolution imaging of large intact volumes of tissue was a new opportunity but created a new challenge. To wit, simple point scanning approaches were translatable to transparent volumes (with the large reduction in light scattering) but were prohibitively slow, and also caused fluorophore bleaching issues since the entire tissue is illuminated as the imaging pinhole is slowly scanned. Fast lightsheet methods cracked this problem, allowing widefield cell-resolution imaging of large transparent volumes with speed suitable for quantitating adult whole-brain cohorts.
-
31.
A further innovation was linking these datasets with activity traces, so that all individual cells (and their brainwide projections) that had been strongly active during a specific experience (for example, a negative-valence mild shock, or a positive valence cocaine administration) could be automatically counted in large cohorts of adult mice. Projections across the brain, such as from mPFC to ventral striatum, could be identified from individual cells that furthermore could be molecularly phenotyped in the same volumes (as with the important immediate-early transcription factor NPAS4).
-
32.
In general, these hydrogel-tissue chemistry methods, by virtue of their all-aqueous-solution implementation and robust tissue-gel composition, afford unique opportunities for rich phenotyping of both proteins and nucleic acids as well as other advantages, including preservation of native fluorophores—the latter particularly important for the goal of registering fluorescent activity signals observed during life with the molecular and wiring information (from the very same cells) that can only be acquired after life.
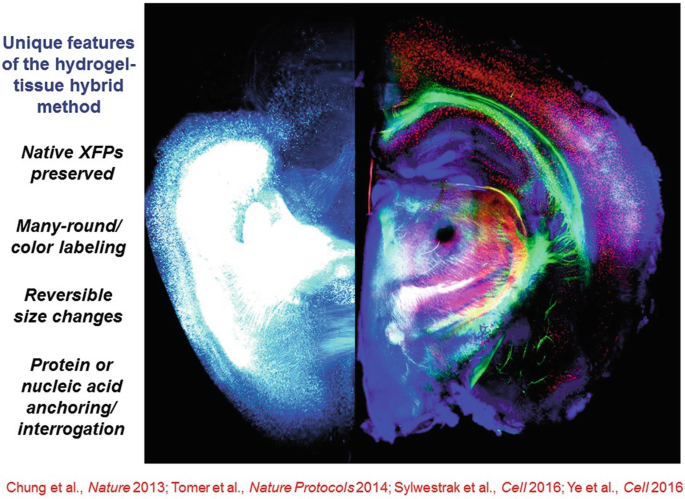
-
33.
While this approach will always be fundamentally designed for basic science, examples of translation of ideas from optical control to the clinic are already occurring. For example, it has become clear in multiple clinical domains that elevated mPFC activity has been associated with reduced reward-seeking or experiencing, both in the context of anhedonia in depression and cocaine seeking in addiction treatment. An optogenetics-guided clinical trial has already found efficacy for treatment in cocaine addiction in studies led by Bonci and colleagues.
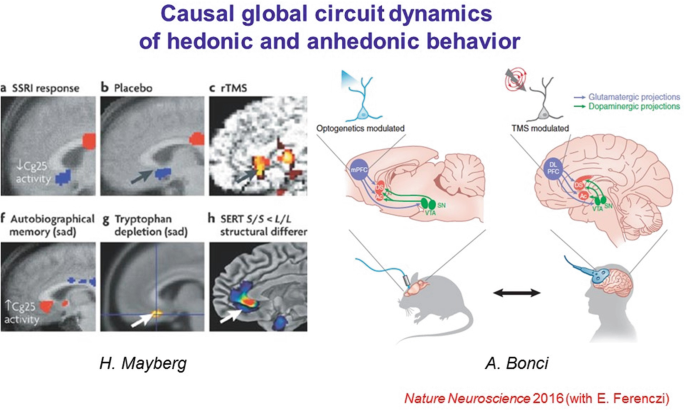
-
34.
Furthermore, the combination of step-function and red-shifted opsins in fact allowed a causal, basic-science test of mechanisms by which elevated mPFC activity might suppress reward experience or motivation. First, the blue-light-driven step-function opsin SSFO was used to stably elevate mPFC activity in freely-moving rats; this intervention was observed to cause anhedonic behavior in these animals.
-
35.
Second, the ventral tegmental area dopamine (VTA-DA) neuron population (source of a primary reward signal in these animals and likely in human beings as well) was optogenetically stimulated (with the red light-driven opsin C1V1 to avoid crosstalk with SSFO circuit elements); it was observed that in those epochs wherein the prefrontal SSFO had been recruited, the stimulated VTA-DA cells were much impaired in their ability to recruit downstream reward circuitry in the ventral striatum. These findings, requiring multiple redesigned microbial opsins to achieve, provided a brainwide circuit-dynamical mechanism for suppression of reward behavior as seen clinically.
-
36.
It is remarkable to consider that some of the most ancient and challenging clinical conditions, such as those in psychiatry which admitted to medical-model description only 200 years ago, are now yielding to mechanistic studies that rely on atomic-scale structure-function studies of microscopic-plant proteins—a testament to basic science.
-
37.
This work required an immensely talented team of students, postdoctoral fellows, esteemed collaborators, staff, and funding agencies, whose names are indicated here.

References
Primer with methods and references linking optogenetics to hydrogel-tissue chemistry and fluorescence activity imaging. https://web.stanford.edu/group/dlab/media/papers/kimNatureReviews2017.pdf
Resource links for hydrogel-tissue chemistry. http://clarityresourcecenter.org/pdfs/Table_S2_Transparency_Methods.pdf
Review on optogenetics. https://web.stanford.edu/group/dlab/media/papers/deisserothNatNeurosciCommentary2015.pdf
Review on channelrhodopsins. https://web.stanford.edu/group/dlab/media/papers/deisserothScience2017.pdf
Online Resources
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Supplementary Electronic Material (S)
(MP4 992920 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Deisseroth, K. (2020). Neural Circuit Dynamics of Brain States. In: Toyama, Y., Miyawaki, A., Nakamura, M., Jinzaki, M. (eds) Make Life Visible. Springer, Singapore. https://doi.org/10.1007/978-981-13-7908-6_5
Download citation
DOI: https://doi.org/10.1007/978-981-13-7908-6_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7907-9
Online ISBN: 978-981-13-7908-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)