Abstract
Calcium oxalate crystals (COC) are one of the most prevalent and widely distributed biomineralizations in plants. The aim of this work is to analyze and compare the data previously reported about the presence and production of COC in leaves of plant species from forests, wetlands, and agroecosystems of the southeast of the Pampean Plain. Diaphanization, clearing of tissues with 50% sodium hypochlorite, and cross sectioning of the leaves were realized. The material was mounted with gelatin–glycerin, and COC were identified and described with optical, polarization, and scanning electron microscopes. Crystal size and density were calculated. Calcification mainly occurred in leaf mesophyll. In terrestrial species, crystals were closely associated with vascular bundles, while in aquatic species, they were associated with aerenchyma. Druses, prisms, and raphides were observed in the leaves of all species analyzed. Average crystal size was smaller in terrestrial species than aquatic ones (12 and 80 μm, respectively), but average crystal density was higher (246 and 23 crystals/mm2, respectively). These different patterns in COC production and distribution may be related to taxonomical characteristics, the types of cells where crystals precipitate, their function, and the differential transpiration rates, among other factors.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Biomineralizations
- Phytoliths
- Calcium oxalate crystals
- Terrestrial and aquatic ecosystems
- Forest
- Agroecosystem
- Pampean plain
1 Introduction
Biomineralizations in plants are called phytoliths (Coe et al. 2014), and calcium oxalate crystals (COC) are one of the most common (Metcalfe 1985). The calcium absorbed from the environment through the roots is combined with oxalate (one of the products of plant metabolism) to produce crystals of different morphologies such as prisms, styloids, raphides, druses, and crystal sand that precipitate within the vacuole of specific cells called idioblasts (Franceschi and Horner 1980; Franceschi and Nakata 2005; Bauer et al. 2011).
COCs play essential structural and physiological functions (Franceschi and Horner 1980; Franceschi and Nakata 2005). COCs serve as a Ca+2 sink and source, preventing Ca accumulation and ensuring the normal cells functions, protect against herbivory and chewing insects, and play structural functions (Ilarslan et al. 1997; Prychid and Rudall 1999; Molano-Flores 2001; Braissant et al. 2004; Franceschi and Nakata 2005; Korth et al. 2006; Bauer et al. 2011). They also have taxonomic importance since their morphology and distribution in plant tissues and organs are characteristic of taxa (Franceschi and Horner 1980; Franceschi and Nakata 2005; Lersten and Horner 2008). Moreover, once in the soil, oxalotrophic bacteria promote COC oxidation because they function as a carbon, energy, and electron source, influencing the carbon and calcium cycle in soils (Braissant et al. 2004; Verrecchia et al. 2006).
In spite of the biological, ecological and biogeochemical importance of COC in plants, there are few researches about the COC description and quantification in relation to the type of community and/or environment, especially in our country. The aim of this work is to analyze and compare the data previously reported about the presence and production of COC in leaves of plant species from forests, wetlands, and agroecosystems of the southeast of the Pampean Plain, Argentina.
2 Materials and Methods
2.1 Study Sites
The southeast of Buenos Aires province (38°12′ S, 57°48′ W) belongs to the geomorphological unit known as “Perinange aeolian hills,” which comprises a relief of morphologically complex hills, with relative heights of up to 30 m and concave–convex profiles with intermediate straight patches and slopes between 6% and 8% (Osterrieth et al. 1998) (Fig. 32.1). The hills originated from processes of primary aeolian accumulation, modified later by superficial wash (Osterrieth and Martínez 1993). In this region, the climate is mesothermic and subhumid, with little or no water deficiency, including an annual precipitation of 809 mm (Burgos and Vidal 1951). Los Padres Basin (37° 55′ 14.47″ and 38° 1′ 50.56″ S, 57° 52′ 21.96″ and 57° 43′ 0.95″ W) is also located in the southeast of the Pampean region. Los Padres wetland is a shallow permanent lake (maximum depth 2.4 m) with an area of 216 ha bounded by the Tandilia Range, a block mountain system (Cionchi et al. 1982). The main processes that produced the wetland were tectonic events and wind erosion (Cionchi et al. 1982). The waterbody receives input from rainwater and groundwater and constitutes open systems recharge–discharge (Cionchi et al. 1982).
Grasslands were the pristine vegetation predominant in the study area across the Quaternary (Cabrera 1976). Approximately 150 years ago, because of the intense agricultural and horticultural activity in the Pampean Plain, these native plant communities had been replaced by crops, where the soybean is one of the most representative (Aizen et al. 2009). Also, at about 50 years ago, a process of artificial forestation with the aim of creating recreation areas generated the introduction of forest species like Eucalyptus sp., Pinus sp., Cupressus sp., and Acacia sp., among others.
2.2 Sample Units
Terrestrial and aquatic vegetation was collected from these environments:
-
(a)
Forest mainly composed of Acacia melanoxylon R. Brown (Fabaceae: Mimosoideae) and Eucalyptus globulus Labill (Myrtaceae), both associated with Celtis ehrenbergiana (Klotzsch) Liebm. (Celtidaceae), a native species.
-
(b)
Agroecosystem with soybean (Glycine max L., Fabaceae: Faboideae) crop.
-
(c)
Los Padres wetland with these dominant aquatic species: Alternanthera philoxeroides (Mart.) Griseb. (Amaranthaceae), Ludwigia peploides (Kunth) P.H. Raven (Onagraceae), Polygonum hydropiperoides Michx. (Polygonaceae), Rumex crispus L. (Polygonaceae), Hydrocotyle bonariensis Lam. (Apiaceae), Typha latifolia L. (Typhaceae).
2.3 Description and Quantification of Calcium Oxalate Crystals
For each species, leaves from at least two plants at flowering or fruiting stage were sampled, washed with distilled water, and cleaned with an ultrasound bath (Test-Lab, TBC 10 model) in order to remove any adhered material. Afterward, tissue clarification (Dizeo de Strittmater 1973) and cross-sectioning were applied. The material was mounted with gelatine–glycerine, and calcium oxalate crystals were identified and described with a petrographic (Olimpus BX 51P) and optical microscope (Leitz Wetzlar D35780) at 400× magnification. Photographs were taken with a Kodak EasyShare CX7530 digital camera.
Average crystals size was calculated from the measuring of about 10–90 crystals per species, depending on the COC production of the species. Crystal density (n° crystals/mm2) was determined within an area of 0.196 mm2 in the clarified leaf samples. Between five and ten areas per leaf were analyzed according to the size of the leaves.
3 Results and Discussion
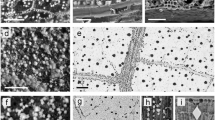
COC were mainly located in parenchyma tissue and randomly distributed in the mesophyll (Fig. 32.2a, f–j). Moreover, in terrestrial species (A. melanoxylon, E. globulus, C. ehrenbergiana, G. max, H. bonariensis), COC were associated to vascular bundles (Fig. 32.2a–e), whereas in some aquatic species (T. latifolia, R. crispus) COC were also distributed in the cells around the air spaces of aerenchyma tissue (Fig. 32.2k) (Graciotto Silva-Brambilla and Moscheta 2001; Jáuregui-Zúñiga et al. 2003; Cervantes-Martínez et al. 2005; Torres Boeger et al. 2007; Borrelli et al. 2009, 2011, 2016). These COC distribution patterns could be related with their different functions. The sequestration of Ca+2 in the COCs lets the normal functioning of chlorenchyma cells and could be also involved in the diffraction of light improving the photosynthesis process (Franceschi and Nakata 2005; Horner 2012). Moreover, the presence of COCs closely associated with vascular bundles in terrestrial species could be explained by their higher transpiration rate. As Ca+2 is distributed along the entire plant via xylem, it is possible that the precipitation of biomineralizations in these areas prevents the mobilization of calcium excess through cells (Prychid and Rudall 1999; Franceschi and Nakata 2005; Lersten and Horner 2008; Gilliham et al. 2011). On the other hand, the differential COC distribution in leaf tissues between terrestrial and aquatic species could be explained by the reduced xylem system and the important aerenchyma tissue characteristic of aquatic plants (Fahn 1990). In addition to the structural function of COCs in the aerenchyma (Kuo-Huang et al. 1994; Prychid and Rudall 1999), the presence of calcium could increase cell wall plasticity around air spaces (Kausch and Horner 1983).
Calcium oxalate crystals in leaves of the terrestrial and aquatic species analyzed. Druses in the mesophyll and prisms associated to vascular bundles in E. globulus (a). Prisms associated with vascular bundles in E. globulus (b), A. melanoxylon (c), and G. max (d–e). Druses and raphides in the mesophyll of L. peploides (f–g). Druses in the mesophyll of P. hydropiperoides (h–i) and R. crispus (j). Druses in the aerenchyma of R. crispus (k). Raphides in T. latifolia (l). vb: vascular bundles. Arrows: COC. Scale bars: 10 μm (a–e, g, i), 100 μm (f, h, j–k)
As it was previously reported, there are differences about COC morphologies between species (Franceschi and Horner 1980; Franceschi and Nakata 2005; Lersten and Horner 2008). Terrestrial species produced druses (E. globulus, C. ehrenbergiana, H. bonariensis) and prisms (E. globulus, A. melanoxylon, G. max) (Fig. 32.2a–e, Table 32.1) (O’Connell et al. 1983; Ilarslan et al. 1997; Borrelli et al. 2009, 2016; He et al. 2012, 2013), while aquatic plants produced druses (R. crispus, P. hydropiperoides, L. peploides, A. philoxeroides) and raphides (T. latifolia, L. peploides) (Fig. 32.2f–l, Table 32.1) (Kausch and Horner 1983; Kuo-Huang et al. 1994; Prychid and Rudall 1999; Graciotto Silva-Brambilla and Moscheta 2001; Lytle 2003; Duarte and Debur 2004; Borrelli et al. 2011). This differential production of morphologies is related to taxonomical characteristics, the types of cells where crystals precipitate and their function (Franceschi and Nakata 2005; Borchert 1984).
Differences were also observed in relation with crystals size and density (Table 32.1). Generally, in terrestrial species, average crystal size was smaller than aquatic species (12 and 80 μm, respectively), but average crystal density was higher (246 and 23 crystals/mm2, respectively) (Table 32.1). This trend could be related with the differential transpiration rates between aquatic and terrestrial species, among others.
In summary, calcium is an important macronutrient necessary for the normal development of plants. COC production let the different species to regulate Ca+2 concentration in cells along with many other physiological and structural functions that enhance the normal cell functioning. Differences in terrestrial and aquatic environments influence the COC production and distribution among leaf tissues.
References
Aizen MA, Garibaldi LA, Dondo M (2009) Expansión de la soja y diversidad de la agricultura Argentina. Ecol Austral 19:45–54
Bauer P, Elbaumb R, Weissc IM (2011) Calcium and silicon mineralization in land plants: transport, structure and function. Plant Sci 180:746–756
Borchert R (1984) Functional anatomy of the calcium excreting system of Gleditsia triacanthos L. Bot Gaz 145:474–482
Borrelli N, Osterrieth M, Marcovecchio J (2009) Calcium biominerals in typical Argiudolls from the Pampean Plain, Argentina: an approach to the understanding of their role within the calcium biogeochemical cycle. Quat Int 193:61–69
Borrelli N, Fernández Honaine M, Altamirano SM, Osterrieth M (2011) Calcium and silica biomineralization in leaves of eleven aquatic species of the Pampean Plain, Argentina. Aquat Bot 94:29–36
Borrelli N, Benvenuto ML, Osterrieth M (2016) Calcium oxalate crystal production and density at different phenological stages of soybean plants (Glycine max L.) from the southeast of the Pampean Plain, Argentina. Plant Biol 18(6):1016–1024
Braissant O, Cailleau G, Aragno M, Verrecchia EP (2004) Biologically induced mineralization in the iroko Milicia excelsa (Moraceae): its causes and consequences to the environment. Geobiology 2:59–66
Burgos JJ, Vidal A (1951) Los climas de la República Argentina según la nueva clasificación de Thornthwaite. Meteor-Forschung 1:3–32
Cabrera AL (1976) Regiones fitogeográficas argentinas. Editorial ACME SACI, Buenos Aires, 85 pp
Cervantes-Martinez T, Horner H, Palmer R, Hymonwitz T, Brown A (2005) Calcium oxalate crystal macropatterns in leaves of species from groups Glycine and Shuteria (Glycininae; Phaseoleae; Papilionoideae; Fabaceae). Can J Bot 83:1410–1421
Cionchi J, Schnack E, Alvarez J, Bocanegra E, Bogliano J, del Río JL (1982) Caracterización hidrogeológica y físico-ambiental preliminar de la Laguna de Los Padres, Partido de General Pueyrredón, provincia de Buenos Aires. Centro de Geología de Costas y del Cuaternario (CGCyC) – Municipalidad de General Pueyrredón (MGP), 100 pp
Coe HH, Osterrieth M, Fernández Honaine M (2014) Phytoliths and their applications. In: Gomes Coe H, Osterrieth M (eds) Synthesis of some phytolith studies in South America. Nova Publishers, New York, pp 1–26
Dizeo de Strittmater CG (1973) Nueva técnica de diafanización. Bol Soc Arg Bot 15:126–129
Duarte MR, Debur MC (2004) Characters of the leaf and stem morpho-anatomy of Alternanthera brasiliana (L.) O. Kuntze, Amaranthaceae. Braz J Pharm Sci 40:85–92
Fahn A (1990) Plant anatomy, 4th edn. Pergamon Press, Oxford
Franceschi VR, Horner HT (1980) Calcium oxalate crystals in plants. Bot Rev 46:361–427
Franceschi V, Nakata P (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56:41–71
Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62:2233–2250
Graciotto Silva-Brambilla M, Moscheta IS (2001) Anatomia foliar de Polygonaceae (Angiospermae) da planície de inundac¸ ão do alto rio Paraná. Maringá 23:571–585
He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo J (2012) Morphologies and elemental compositions of calcium crystals in phyllodes and branchlets of Acacia robeorum (Leguminosae: Mimosoideae). Ann Bot 109:887–896
He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo J (2013) Precipitation of calcium, magnesium, strontium and barium in tissues of four Acacia species (Leguminosae: Mimosoideae). PLoS One 7:e41563
Horner HT (2012) Peperomia leaf cell wall interface between the multiple hypodermis and crystal-containing photosynthetic layer displays unusual pit fields. Ann Bot 109:1307–1315
Ilarslan H, Palmer RG, Imsande J, Horner HT (1997) Quantitative determination of calcium oxalate and oxalate in developing seeds of soybean (Leguminosae). Am J Bot 84:1042–1046
Jáuregui-Zúñiga D, Reyes-Grajeda JP, Sepulveda-Sanchez JD, Whitaker JR, Moreno A (2003) Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifera. Plant Physiol 160:239–245
Kausch AP, Horner HT (1983) The development of mucilaginous raphide crystal idioblasts in young leaves of Typha angustifolia L. (Typhaceae). Am J Bot 70:691–705
Korth K, Doege SJ, Park S-H, Goggin FL, Wang Q, Gomez SK, Liu G, Jia L, Nakata PA (2006) Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol 141:188–195
Kuo-Huang LL, Chiou-Rong S, Yuen-Po Y, Shu-Hwa TC (1994) Calcium oxalate crystals in some aquatic angiosperms of Taiwan. Bot Bull Acad Sin 34:179–188
Lersten NR, Horner HT (2008) Crystal macropatterns in leaves of Fagaceae and Nothofagaceae: a comparative study. Plant Syst Evol 271:239–253
Lytle ST (2003) Adaptation and acclimation of populations of Ludwigia repens to growth in high-and lower-CO2 springs. Dissertation, University of Florida, 157pp
Metcalfe CR (1985) Secreted mineral substances. Crystals. In: Metcalfe CR, Chalk L (eds) Anatomy of the Dicotyledons II. Word structure and conclusion of the general introduction. Clarendon Press, Oxford, pp 82–97
Molano-Flores B (2001) Herbivory and calcium concentrations affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann Bot 88:387–391
O’Connell AM, Malajczuk N, Gailitis V (1983) Occurrence of calcium oxalate in Karri (Eucalyptus diversicolor F, Muell.), Forest ecosystems of South Western Australia. Oecología (Berlin) 56:239–244
Osterrieth M, Martínez G (1993) Paleosols on late Cainozoic sequences in the northeastern side of tandilia range, Buenos Aires, Argentina. Quat Int 17:57–65
Osterrieth M, Fernández C, Bilat Y, Martínez P, Martínez G, Trassens M (1998) Geoecología de Argiudoles típicos afectados por prácticas hortícolas en la Llanura Pampeana, Buenos Aires, Argentina. Abstracts XVI Congreso Mundial de la Ciencia del Suelo, pp 1–8
Prychid CJ, Rudall PJ (1999) Calcium oxalate crystals in monocotyledons: a review of their structure and systematics. Ann Bot 84:725–739
Torres Boeger MR, Barbosa de Oliveira Pil MW, Filho NB (2007) Arquitetura foliar comparativa de Hedychium coronarium J. Koenig (Zingiberaceae) ede Typha domingensis Pers (Typhaceae). Iheringia, Sér Bot Porto Alegre 62:113–120
Verrecchia EP, Braissant O, Cailleau G (2006) The oxalate-carbonate pathway in soil carbon storage: the role of fungi and oxalotrophic bacteria. In: Gadd M (ed) Fungi in biogeochemical cycles. Cambridge Univ. Press, Cambridge, pp 289–310
Acknowledgments
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 1583/2013), the Universidad Nacional de Mar del Plata (EXA 741/15), and CONICET PIP (11220130100145CO).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this paper
Cite this paper
Altamirano, S.M., Borrelli, N., Benvenuto, M.L., Honaine, M.F., Osterrieth, M. (2018). Calcium Oxalate Crystals in Plant Communities of the Southeast of the Pampean Plain, Argentina. In: Endo, K., Kogure, T., Nagasawa, H. (eds) Biomineralization. Springer, Singapore. https://doi.org/10.1007/978-981-13-1002-7_32
Download citation
DOI: https://doi.org/10.1007/978-981-13-1002-7_32
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1001-0
Online ISBN: 978-981-13-1002-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)