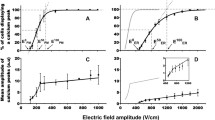
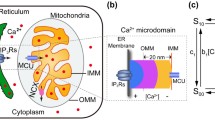
Abstract
The endoplasmic reticulum (ER) is a tubular membranous labyrinth throughout the cytoplasm that is continuous with the nuclear membrane, exhibits contact sites with mitochondria and the plasma membrane, and displays domains for specific functions. It is smooth or rough with ribosomes for protein synthesis and is the home for folding proteins in their proper tertiary structures. The ER includes cellular stress response sensors that can lead to unfolded protein response (UPR), leading to regulated cell death (RCD). The ER is also a primary storage site for Ca2+ that can be used for scores of Ca2+ mediated signal transduction responses such as neurotransmitter release, muscle contraction, or contributors to RCD, among others. Since usEPs were unique for intracellular electric field effects, usEP-induced Ca2+ release was an excellent way to define these intracellular effects, albeit not without caveats. Because usEPs also induced plasma membrane permeabilization for Ca2+ influx, which occurred at lower charging conditions than ER-induced nanopore formation and Ca2+ release; because some cells expressed voltage-gated Ca2+ channels (VGCC), which could be directly activated or activated due to usEP-induced plasma membrane depolarization; because capacitative Ca2+ increases could increase intracellular Ca2+; and Ca2+ increases from Ca2+-induced Ca2+ release, significant care, and experimental manipulations were required to be sure that increases in intracellular Ca2+ were due to release from internal stores. Also, because there were other intracellular stores for Ca2+, other approaches were needed to ensure that the source of intracellular Ca2+ release was from the ER. This chapter provides details from several studies using many different experimental techniques that lead to the conclusion that usEPs could induce Ca2+ release from the ER by forming ER nanopores. Notably, another series of experimental studies supported theoretical evidence that shorter pulse durations lead to more significant increases in intracellular Ca2+ than longer pulse durations.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alberts B, Johnson A, Lewis L, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell. Garland Science, New York
Alemany R, Sichelschmidt B, Zu Heringdorf DM, Lass H, van Koppen CJ, Jakobs KH (2000) Stimulation of sphingosine-1-phosphate formation by the P2Y(2) receptor in HL-60 cells: Ca(2+) requirement and implication in receptor-mediated Ca(2+) mobilization, but not MAP kinase activation. Mol Pharmacol 58(3):491–497
Beebe SJ (2013) Cell responses without receptors and ligands, using nanosecond pulsed electric fields (nsPEFs). Int J Nanomed 8:3401–3404
Beebe SJ (2015) Considering effects of nanosecond pulsed electric fields on proteins. Bioelectrochemistry 103:52–59
Beebe SJ, White JA, Blackmore PF, Deng Y, Somers K, Schoenbach KH (2003) Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol 22:785–796
Beebe SJ, Chen YJ, Sain NM, Schoenbach KH, Xiao S (2012) Transient features in nanosecond pulsed electric fields differentially modulate mitochondria and viability. PLoS ONE 7(12):e51349
Beebe SJ, Sain NM, Ren W (2013) Induction of cell death mechanisms and apoptosis by nanosecond pulsed electric fields (nsPEFs). Cells 2(1):136–162
Berman MC (2000) Characterisation of thapsigargin-releasable Ca(2+) from the Ca(2+)-ATPase of sarcoplasmic reticulum at limiting (Ca(2+)). Biochim Biophys Acta 1509(1–2):42–54
Berridge MJ (1998) Neuronal calcium signaling. Neuron 21(1):13–26
Berridge MJ (2009) Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793(6):933–940
Buescher ES, and Schoenbach KH (2003) Submicrosecond intense pulsed electric field effects on intracellular free calcium: mechanisms and effects. IEEE Trans Dielectrics Electr Insul 10:788–794
Buescher ES, Smith RR, Schoenbach KH (2004) Submicrosecond intense pulsed electric field effects on intracellular free calcium: mechanisms and effects. IEEE Trans Plasma Sci 32:1563–1572
Craviso GL, Choe S, Chatterjee I, Vernier PT (2012) Modulation of intracellular Ca2+ levels in chromaffin cells by nanoelectropulses. Bioelectrochemistry 87:244–252
Crimaudo C, Hortsch M, Gausepohl H, Meyer DI (1987) Human ribophorins I and II: the primary structure and membrane topology of two highly conserved rough endoplasmic reticulum-specific glycoproteins. EMBO J 6(1):75–82
Dean WL, Whiteheart SW (2004) Plasma membrane Ca(2+)-ATPase (PMCA) translocates to filopodia during platelet activation. Thromb Haemost 91(2):325–333
Demonbreun AR, McNally EM (2016) Plasma membrane repair in health and disease. Curr Top Membr 77:67–96
Dobrydneva Y, Blackmore P (2001) 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol Pharmacol 60:541–552
Eisner DA, Caldwell JL, Kistamás K, Trafford AW (2017) Calcium and excitation-contraction coupling in the heart. Circ Res 121(2):181–195
English AR, Voeltz GK (2013) Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol 5(4):a013227
Gowrishankar TR, Esser AT, Vasilkoski Z, Smith KC, Weaver JC (2006) Microdosimetry for conventional and supra-electroporation in cells with organelles. Biochem Biophys Res Commun 341(4):1266–1276
Guo S, Burcus NI, Hornef J, Jing Y, Jiang C, Heller R, Beebe SJ (2018) Nano-pulse stimulation for the treatment of pancreatic cancer and the changes in immune profile. Cancers (Basel) 10(7):217
Harper JL, Camerini-Otero CS, Li AH, Kim SA, Jacobson KA, Daly JW (2003) Dihydropyridines as inhibitors of capacitative calcium entry in leukemic HL-60 cells. Biochem Pharmacol 65(3):329–338
Hristov K, Mangalanathan U, Casciola M, Pakhomova ON, Pakhomov AG (2018) Expression of voltage-gated calcium channels augments cell susceptibility to membrane disruption by nanosecond pulsed electric field. Biochim Biophys Acta Biomembr 1860(11):2175–2183
Kotnik T, Miklavcic D (2006) Theoretical evaluation of voltage inducement on internal membranes of biological cells exposed to electric fields. Biophys J 90:480–491
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Review Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2(11):a003996
Liu Y, Zhu X (2017) Endoplasmic reticulum-mitochondria tethering in neurodegenerative diseases. Transl Neurodegener 6:21
Martens SL, Roth CC, Ibey BL (2018) Nanosecond pulsed electric field exposure does not induce the unfolded protein response in adult human dermal fibroblasts. Bioelectromagnetics 39(6):491–499
Neve EPA, Ingelman-Sundberg M (2010) Cytochrome P450 proteins: retention and distribution from the endoplasmic reticulum. Curr Opin Drug Discov Devel 13(1):78–85
Nuccitelli R, Berridge JC, Mallon Z, Kreis M, Athos B, Nuccitelli P (2015) Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS ONE 10(7):e0134364
Pakhomov AG, Bowman AM, Ibey BL, Andre FM, Pakhomova ON, Schoenbach KH (2009) Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem Biophys Res Commun 385(2):181–186
Pakhomov AG, Xiao S, Pakhomova ON, Semenov I, Kuipers MA, Ibey BL (2014) Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry 100:88–95
Rossi A, Pakhomova ON, Mollica PA, Casciola M, Mangalanathan U, Pakhomov AG, Muratori C (2019) Nanosecond pulsed electric fields induce endoplasmic reticulum stress accompanied by immunogenic cell death in murine models of lymphoma and colorectal cancer. Cancers (Basel) 11(12):2034
Schooley A, Vollmer B, Antonin W (2012) Building a nuclear envelope at the end of mitosis: coordinating membrane reorganization, nuclear pore complex assembly, and chromatin de-condensation. Chromosoma 121(6):539–554
Semenov I, Xiao S, Pakhomova ON, Pakhomov AG (2013) Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: the impact of pulse duration. Cell Calcium 54(3):145–150
Semenov I, Xiao S, Pakhomov AG (2013) Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim Biophys Acta 1828(3):981–989
Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M (2015) Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim Biophys Acta 1853(9):1980–1991
Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr (2006) Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763(11):1147–1160
Stacey M, Fox P, Buescher S, Kolb J (2011) Nanosecond pulsed electric field induced cytoskeleton, nuclear membrane and telomere damage adversely impact cell survival. Bioelectrochemistry 82(2):131–134
Taylor CW (2006) Store-operated Ca2+ entry: a stimulating store. Trends Biochem Sci 31:597–601
Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA 87(7):2466–2470
Tolstykh GP, Beier HT, Roth CC, Thompson GL, Payne JA, Kuipers MA, Ibey BL (2013) Activation of intracellular phosphoinositide signaling after a single 600 nanosecond electric pulse. Bioelectrochemistry 94:23–29
Traaseth N, Elfering S, Solien J, Haynes V, Giulivi C (2004) Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim Biophys Acta 1658(1–2):64–71
Vacca VM Jr (2016) CJD: Understanding Creutzfeldt-Jakob disease. Nursing 46(3):36–42
Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J (2011) The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3:a004184
Verkhratsky A, Shmigol A (1996) Calcium-induced calcium release in neurones. Cell Calcium 19(1):1–14
Vernier PT, Sun Y, Marcu L, Salemi S, Craft CM, Gundersen MA (2003) Calcium bursts induced by nanosecond electric pulses. Biochem Biophys Res Commun 310(2):286–295
Vernier PT, Sun Y, Chen MT, Gundersen MA, Craviso GL (2008) Nanosecond electric pulse-induced calcium entry into chromaffin cells. Bioelectrochemistry 73(1):1–4
Wan B, LaNoue KF, Cheung JY, Scaduto RC Jr (1989) Regulation of citric acid cycle by calcium. J Biol Chem 264(23):13430–13439
Wang S, Chen J, Chen MT, Vernier PT, Gundersen MA, Valderrábano M (2009) Cardiac myocyte excitation by ultrashort high-field pulses. Biophys J 96(4):1640–1648
White JA, Blackmore PF, Schoenbach KH, Beebe SJ (2004) Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J Biol Chem 279(22):22964–22972
Wilson CM, Roebuck Q, High S (2008) Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core. Proc Natl Acad Sci USA 105(28):9534–9539
Xia M, Zhang Y, Jin K, Lu Z, Zeng Z, Xiong W (2019) Communication between mitochondria and other organelles: a brand-new perspective on mitochondria in cancer. Cell Biosci 9:27
Zakharov SI, Smani T, Dobrydneva Y, Monje F, Fichandler C, Blackmore PF, Bolotina VM (2004) Diethylstilbestrol is a potent inhibitor of store-operated channels and capacitative Ca(2+) influx. Mol Pharmacol 66(3):702–707
Zehnder JL, Leung LLK (1990) Blood 76:2011–2016
Zhang J, Blackmore PF, Hargrave BY, Xiao S, Beebe SJ, Schoenbach KH (2008) Nanosecond pulse electric field (nanopulse): a novel non-ligand agonist for platelet activation. Arch Biochem Biophys 471(2):240–248
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Beebe, S.J. (2021). usEP Effects on the Endoplasmic Reticulum (ER). In: Ultrashort Electric Pulse Effects in Biology and Medicine. Series in BioEngineering. Springer, Singapore. https://doi.org/10.1007/978-981-10-5113-5_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-5113-5_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5112-8
Online ISBN: 978-981-10-5113-5
eBook Packages: EngineeringEngineering (R0)