Abstract
The border ocelli and adjacent parafocal elements are among the most diverse and finely detailed features of butterfly wing patterns. The border ocelli can be circular, elliptical, and heart-shaped or can develop as dots, arcs, or short lines. Parafocal elements are typically shaped like smooth arcs but are also often “V,” “W,” and “M” shaped. The fusion of a border ocellus with its adjacent parafocal element is a common response to temperature shock and treatment with chemicals such as heparin and tungstate ions. Here I develop a new mathematical model for the formation of border ocelli and parafocal elements. The models are a reaction-diffusion model based on the well-established gradient-threshold mechanisms in embryonic development. The model uses a simple biochemical reaction sequence that is initiated at the wing veins and from there spreads across the field in the manner of a grass-fire. Unlike Turing-style models, this model is insensitive to the size of the field. Like real developmental systems, the model does not have a steady state, but the pattern is “read out” at a point in development, in response to an independent developmental signal such as a pulse of ecdysone secretion, which is known to regulate color pattern in butterflies. The grass-fire model reproduces the sequence of Distal-less expression that determines the position of eyespot foci and also shows how a border ocellus and its neighboring parafocal element can arise from such a single focus. The grass-fire model shows that the apparent fusion of ocellus and parafocal element is probably due to a premature termination of the normal process that separates the two and supports the hypothesis that the parafocal element is the distal band of the border symmetry system.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The color patterns of butterflies are extremely diverse, and almost all of the 14,000 or so species can be identified on the basis of their color patterns alone. Adding to this diversity is the fact that dorsal and ventral color patterns are usually entirely different and that many species have polymorphic, sexually dimorphic, and seasonally plastic color patterns. The development and evolution of this diversity of patterns has been of considerable interest, particularly in relation to the genetics and evolution of mimicry (Reed et al. 2011; Nadeau 2016; Baxter et al. 2008; Joron et al. 2006), and the development and evolution of eyespot patterns (Brakefield et al. 1996; Monteiro et al. 1997, 2003; Monteiro 2015; Nijhout 1980).
The organizing principles of color patterns are coming to be increasingly well understood. The diversity of mimicry patterns in Heliconius butterflies is due to the variation in only a handful of genes (Nadeau 2016; Kapan et al. 2006), and the specification of color and pattern is now known to be due to a redeployment of many of the genes involved in early embryonic development (Carroll et al. 1994; Martin and Reed 2014; Reed and Serfas 2004; Brunetti et al. 2001).
The developmental mechanism that produces the spatial pattern of pigments that characterizes color patterns is less well understood. It is clear, however, that the wing veins and the wing margin play critical roles in organizing the pattern. This evidence comes, among others, from observations of the color patterns of mutants that lack wing veins and from experimental manipulations that alter the wing margin (e.g., Fig. 1.1 and (Nijhout and Grunert 1988; Koch and Nijhout 2002)).
Color pattern modification in the veinless mutant of Papilio xuthus (right), compared with the normal pattern (left). The longitudinal veins are missing and so are the venous patterns. The submarginal bands are smoothly continuous and parallel to the wing margin, suggesting that the wing margin also plays an important role in color pattern determination
2 Eyespots and Parafocal Elements
The color patterns of butterflies are organized as a set of three-symmetry systems (Süffert 1929; Schwanwitsch 1924, 1929; Nijhout 1991). The basal symmetry system is often absent or represented only by its distal band. The central symmetry system runs in the middle region of the wing and is centered on the discal spot. The border symmetry system runs along the distal region of the wing usually paralleling the wing margin (Fig. 1.2). The most complex patterns are typically found in the border symmetry system. The principal elements of the border symmetry system are the border ocelli or eyespots. Although the canonical morphology of an ocellus is a set of concentric circles of contrasting pigments with a well-defined central spot called the focus (Nijhout 1980), circular elements are actually quite uncommon within the larger diversity of butterfly color patterns. More often the shape of the “ocellus” deviates significantly from the circular (heart shaped, dagger shaped, bar shaped) and is often hardly recognizable as homologous to a circular element (Nijhout 1990, 1991).
The nymphalid ground plan showing three symmetry systems: basal, central, and border. The border symmetry system has border ocelli (bo) on the compartment midlines. These border ocelli can develop into elaborate eyespots but also into many other shapes. The shape of the distal band of the border symmetry system can also be very diverse, and this band is recognized as the parafocal element
The proximal and distal bands of the border symmetry system have very different characters. The proximal band, when present, is typically arc shaped, or nearly straight. The distal bands are almost always present and have an exceptionally diverse array of shapes. Because its development and evolution are quite independent of that of the border ocelli, this element has been given a special name: the parafocal element (Nijhout 1990). Süffert (1929) recognized this as the distal band of the border symmetry system but did not give it a special name, and Schwanwitsch (1924) thought it was actually part of the submarginal band system. The results given below in this paper support Süffert’s interpretation, as does the recent work of Otaki and colleagues (Dhungel and Otaki 2009; Otaki 2009, 2011). The parafocal elements are developmentally closely related to the border ocelli. Indeed the two are developmentally interdependent in that they appear to arise from a common determination mechanism, although the determinants of their shape are quite different.
3 Puzzling Results of Temperature Shock Experiments
A number of investigators have observed that when color pattern aberrations are induced by temperature shock and various chemicals, one of the commonly observed features is a partial or complete fusion of the ocellus and the parafocal element (Otaki 2008; Nijhout 1985, 1991; Nijhout and Grunert 1988). The smooth fusion of these two pattern elements (Fig. 1.3) suggests that that must share a common developmental mechanism. If we interpret the series shown in Fig. 1.3 in reverse order, then it would seem that a single pattern element breaks into two, with the distal one forming the parafocal element and the proximal one the ocellus. None of the current models of color pattern formation can account for this.
Fusion of ocelli and parafocal elements after temperature shock in Vanessa cardui. Top row, dorsal surface. Bottom row, ventral surface. Normal patterns are on the left in each row. Bottom row shows a moderately affected pattern in the middle, and a severely affected pattern in which both pattern elements are completely fused is on the right
4 Models of Color Pattern Formation
Previous models for color pattern formation in butterflies have shown that it must be a two-step process. The first step is the establishment of organizing centers, and the second step is the organization of patterns of pigment synthesis by signals produced by these organizing centers. The best known of these organizing centers is the focus, a group of cells that occurs at the center of a canonical eyespot. The foci express both notch and Distal-less, in succession (Carroll et al. 1994; Reed and Serfas 2004), followed by the expression of Spalt and Engrailed in their surrounding, corresponding to the presumptive colored regions of the eyespot (Zhang and Reed 2016; Brunetti et al. 2001).
The mechanism that determines the placement of foci on the wing is still unknown. Foci always occur exactly on the midline of wing compartments delineated by wing veins (i.e., equidistant from the veins). Intervenous stripe patterns (e.g., Fig. 1.6) also occur exactly along the midlines of wing compartments, and in certain papilionids, these stripes break up into spot-like patterns (Nijhout 1991), suggesting a common developmental origin of stripes and spots.
Color pattern determination begins in the wing imaginal disk shortly after the wing venation system is established. The wing imaginal disk is composed of two cell layers, for the dorsal and ventral wing surfaces, respectively. The two cell layers are tightly adhered to each other via a basement membrane. Wing veins develop as tube-like separations between the two layers. The veins are continuous with the hemocoel and allow entry of hemolymph into the developing and growing wing. A special vein called the bordering lacuna (Nijhout 1991) develops around the periphery of the wing imaginal disk and connects the end points of the wing veins (Fig. 1.4).
The wing veins are bordering lacunae and are the only structural elements in the wing disk when pattern formation begins, and theoretical models of pattern formation assume that these structural elements are the first initiators or organizers for pattern development because they are the only way in which developmental signals can enter the wing (an idea supported by pattern aberrations in veinless mutants (Fig. 1.1). Pattern development including placement of the organizing centers must somehow depend on signals arising from the wing veins and bordering lacuna.
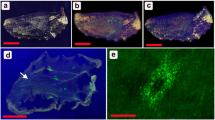
A successful theoretical model for the placement of foci was based on Turing-like reaction diffusion (Turing 1952) using kinetics developed by Meinhardt (1982). The model assumes that a pattern is produced by two chemicals, an autocatalytic activator and an inhibitor, which control each other’s synthesis, which can diffuse freely from cell to cell, and in which the inhibitor acts over a larger distance than the activator. Starting with a system at steady state, introducing a small amount of activator from the wing veins, results in a spatial pattern of activator production that rises first as a stripe along the midline between two wing veins in the distal portion of the wing compartment. The end of this midline stripe becomes a particularly strong source of activator production and gradually represses the rest of the stripe, resulting in a stable point-like pattern on the midline resembling the position of a focus. The exact position of the focus as well as the number of foci produced depends on boundary conditions, size of the field, and parameter values of the reaction scheme. This model gained support from the finding that it predicted the spatial sequence of expression of the gene Distal-less, one of the early determinants of color pattern, almost precisely (Fig. 1.5) (Nijhout 1990, 2010).
Time series of the development of the expression pattern of Distal-less in the imaginal wing disk of Junonia coenia. Black arrows indicate the position of wing veins. White arrows point to the developing stalks and spots of the Distal-less. Initially Distal-less is expressed along the wing veins and wing margin (Plate 1), but then the expression becomes gradually concentrated to the wing compartment midline (Plates 2–5). A spot develops at the tip of the midline bar in wing compartment that will develop an ocellus, and the midline bar gradually disappears (Plates 6–7)
The spatial pattern of point-like foci and various line-like distributions of the presumptive activator is then used in the second stage of pattern formation to induce the synthesis of specific pigments. A simple diffusion-threshold mechanism using these activator distributions as the origins of new diffusible morphogens proved sufficient to explain almost the entire diversity of color patterns found in the butterflies (Nijhout 1990).
There is, however, a significant problem with this model and, in particular, with the reaction-diffusion mechanism that sets up the initial prepattern of activator distributions. Reaction-diffusion mechanisms are notoriously sensitive to field size and to the exact choice of parameter values and boundary conditions. Even small changes in any of these factors can produce extremely different spatial patterns of activator distribution. Reaction-diffusion mechanisms are particularly sensitive to the size of the field and produce wildly different patterns in fields of different sizes. This seems biologically unrealistic. Biological systems tend to be quite robust to parameter variation and size variation, such as produced by the abundant and often severe genetic and environmental variation to which organisms are subject (Nijhout 2002). In particular, in butterflies, identical patterns often develop in adjoining wing compartments of very different dimensions. Finally, although Turing-style reaction-diffusion mechanisms can be made to produce a wide diversity of realistic patterns, there are, with the possible exception of some fish pigment patterns, no instances in which they have been experimentally proven to operate during development and in which the activator and inhibitor have been identified (Kondo and Miura 2010).
This has led me to search for a simpler and more robust mechanism that could produce the diversity of color patterns observed. Developmental genetic studies of embryonic development have revealed a broad array of gene regulatory networks that produce dynamically changing spatial patterns of gene expression, in which the product of one gene acts as a transcriptional regulator of one or more other genes. The effect of a gene spreads either by diffusion of the gene product to adjoining cells or by cell-surface signaling interaction among neighboring cells.
These mechanisms for pattern formation are conceptually and physically simple. They are in effect diffusion-threshold mechanisms, in which a substance diffuses away from the cells where it is produced and exerts its effect when it rises above a threshold in surrounding cells. These diffusion-threshold mechanisms can be generalized into what I’ll call a grass-fire model.
5 The Grass-Fire Model
The model consists of the simplest possible set of reactions. A molecule we will call fuel is initially distributed across the field and serves as substrate for the first reaction to produce the product P1. P1 in turn serves as the substrate for the production of P2 and so forth. The model is given by:
Initially there is only fuel, and the patterning mechanism is initiated when P1 is introduced at some point in the field, for instance, along the margins of the field. The model resembles a grass-fire with a fire front, initiated at the ignition point where P1 is introduced, that consumes fuel and leaves combustion products behind, some of which can be used in other reactions. In addition to these reactions, we assume that all chemicals can diffuse from areas of high concentration to low concentration. We assume for the present that all reactions are mass action. Thus we have an exceptionally simple reaction-diffusion system.
In the course of time fuel is depleted, as are all subsequent metabolites. This system does not produce a stable end pattern but rather a slowly changing spatial pattern of values of the three variables. In this respect it resembles the early gene expression patterning events in the Drosophila embryo in which a successive series of diffusion gradient-threshold events produce a dynamically progressing spatial pattern of gene expression (Tomancak et al. 2002). We assume that an independent event “reads” the spatial pattern of chemicals at some time point in the development. In butterflies this could be the ecdysone signals that initiate a molt or the wandering stage, both of which occur during the period of color pattern formation and also control growth and morphogenesis of the wing imaginal disk.
The nature of fuel, P1 and P2, is undetermined. Any system with mass action kinetics will do, nor are the kinds of kinetics restricted to mass action. Saturation kinetics like Michaelis-Menten and Hill produce the same patterns as mass-action kinetics over a range of parameter values. The reactions could therefore represent a biochemical reaction sequence, a gene activation sequence, a successive activation of signaling cascades, or a combination of these.
6 Basic Patterns
We assume the field is a rectangle that represents a compartment in the wing imaginal disk, where the top and long sides are wing veins and the bottom short side is the bordering lacuna. The reactions can be initiated only along these edges. Variation in pattern can come about by a variation in the position of the initiation points (along the entire margin or only near the proximal, middle, or distal ends), the initial distribution of fuel (homogeneous, proximodistal gradient, vein to midline gradient), and the distribution of the enzymes or rate constants, that run the reactions (homogeneous, proximodistal gradient, vein-to-midline gradient).
7 Venous and Intervenous Patterns
Some of the simplest and most widespread patterns are stripes that run along the midline of a compartment and patterns that run parallel to the wing veins. Figure 1.6 illustrates several examples. The patterns show that the veins do not induce pattern along their entire length. In Fig. 1.6a the pattern is only induced in the mid-region of the vein but not near the proximal and distal ends. There is often a proximodistally graded width of the venous bands suggesting (e.g., Fig. 1.6d–e) that the strength of induction, or the propagation rate of the inductive signal, is graded. These patterns are readily produced by the grass-fire model, as illustrated in Fig. 1.7. A proximodistal gradient of reaction rate constants produces venous bands that taper along the length of the vein (Fig. 1.7c). Intervenous stripes (Fig. 1.7a) can be made if the entire wing vein induces the pattern and both the fuel and reaction rates are homogeneously distributed. Reed and Serfas (2004) have shown that in butterflies without eyespots, but with intervenous stripes, there is a long central midline stripe of notch and Distal-less expression. Notch and Distal-less also specify the position of eyespot foci (see below), thus the patterns of P1 and P2 may simulate the expression of these two peptides.
Vein-dependent patterns. Top row shows venous patterns of Anaxita decorata (a) and intervenous patterns of Pseudacraea lucretia (b) and Eteone eupolis (c). Bottom row (d–f) shows individual variation in Danaus affinis. In Danaus the white venous pattern varies in the extent to which it expands from the wing veins
8 Simulation of Notch and Distal-Less Progression
The progression of Distal-less expression (Fig. 1.5), beginning with a short midline stripe of the emerging from the margin, followed by the development of a spot at the apex of the stripe, followed by a regression of the stripe, leaving a spot of Distal-less expression behind, was accurately reproduced by a Turing-style reaction diffusion program (Nijhout 1990). Indeed, it provided strong, albeit circumstantial, support for reaction diffusion as the underlying mechanism of focus formation.
Reed and Serfas (2004) and Zhang and Reed (2016) have shown that this pattern of Distal-less expression is preceded by an almost identical pattern of notch expression.
The grass-fire model produces both pattern sequences (Fig. 1.8), simply by assuming that only the distal portion of the wing veins acts as initiation sources and that the fuel is distributed in a shallow gradient that is higher near the midline than near the veins. The shape of the focal spot is slightly elongated across the long axis of the wing compartment, just as the expression of notch and Distal-less described by Reed and Serfas (2004) and Zhang and Reed (2016). The pattern of P2 is identical to that of P1 but lags behind a little, and P2 still has a stalk when P1 is already resolved into a spot (Fig. 1.8). Thus the progression of P1 and P2 resemble those of notch and Distal-less, respectively.
Model simulations of focus formation. Two runs are shown with slightly different initial distributions of “fuel.” The distributions of P1 and P2 are shown, which could correspond to the notch and Distal-less, respectively. The two patterns differ in the shape of the lateral gradient of the “stalk,” which affects the shape of the parafocal element that will develop
9 Shape of the Parafocal Elements
As noted above, once the foci are established, the second step in color pattern formation is a signal that originates from the foci and that specifies a pattern of pigment biosynthesis in their surroundings. We use the grass-fire model for this second step as well, using the focus as the initiation point.
If the grass-fire model is started from a single point source, the pattern produced naturally breaks into two fronts, moving distally and proximally, respectively. If the initial substrate that is used is homogeneously distributed, a circular pattern will form that breaks into two semicircular arcs that move away from the initiation point.
A characteristic feature of the parafocal elements is that they are always symmetrical around the wing compartment midline and are often Λ, V, W, or M shaped (e.g. Fig. 1.9), suggesting a special function of the midline in shaping this element. If the parafocal element is formed by a moving reaction front, then movement near the midline and/or the veins must be either more rapid or slower than movement elsewhere. One way to accomplish this is by having a required metabolite or precursor to the reaction distributed in a pattern that is symmetrical to the midline. A clear candidate for this is the gradient left behind by the midline pattern that preceded the formation of the focal spot (Fig. 1.8). This midline concentration gradient decays only gradually, and its profile depends on the parameter values and initial fuel distribution.
The hypothesis then is that the shape of the parafocal elements is determined by a gradient left behind by the process that formed the focus. This idea can be tested computationally. Figures 1.10a and 1.11 show a sample of the diversity of parafocal element shapes that can be produced by this model. Although these shapes closely mimic those of real parafocal elements (e.g., Fig. 1.9), the shape of the ocellus is not circular, as would typically be the case.
Simulations of pattern generated by focal sources. (a) A single source breaks up into an ocellus and a parafocal element, but the ocellus is not circular. (b–g) A double source at the focus, one producing the eyespot (b) and the other producing the parafocal element and the proximal arc-shaped band of the border symmetry system (c–g)
To produce both perfectly circular eyespots and the right diversity of parafocal element shapes, it is necessary to assume that the focus could be the source of two different signals (one perhaps initiated by notch and the other by Distal-less) that use different substrates. If one signal uses a homogeneously distributed substrate, it will produce a circular eyespot (Fig. 1.10b), and if the other uses the gradient left behind by the focus-forming process, it produces the parafocal element. Interestingly, this second source also produces an arc-shaped pattern on the proximal side of the eyespot (Fig. 1.10c–g). This finding is consistent with Süffert’s idea that the parafocal element is the distal band of the border symmetry system: the parafocal element and the proximal arc produced by the second source make up paired bands of the border symmetry system. These model results also support the ideas about the nature of parafocal elements and border symmetry systems proposed by Otaki (Dhungel and Otaki 2009; Otaki 2009, 2011).
10 Fusion and Separation of Ocelli and Parafocal Elements
When the pupae of butterflies are exposed to a temperature shock, many individuals exhibit a fusion between the ocellus and the parafocal element. The degree of fusion is quite variable from individual to individual, and in extreme cases the two fuse into a single pattern element (Fig. 1.3). A possible reason for this effect is that temperature shock freezes the progression of pattern determination, possibly by activating heat shock or stress proteins that stop biosynthetic or transcriptional activity (Mitchell and Lipps 1978; Crews et al. 2016; Welte et al. 1995). The grass-fire model shows that a single pattern element can split into two and that both ocelli and parafocal elements can be produced from a common source.
11 Modes of Pattern Evolution
The developing pattern depends on only a few variables: the kinetic parameters of the reactions and the initial gradients of fuel. For all models explored here, these gradients are simple. Beside homogeneous distributions, we used smooth proximodistal gradients or smooth gradients symmetrical to the wing compartment midline, parallel to the wing veins. The latter could be readily set up by diffusion from, or absorption by, the wing veins. Thus the anatomical features of the wing, the wing veins and bordering lacunae, are the only features used to initiate pattern formation.
A significant way in which the proposed patterning mechanism differs from the assumptions of a typical Turing-style reaction-diffusion mechanism is that the system is never at steady state, but the pattern slowly changes over time. The developing pattern becomes fixed, so to speak, by an event such as a pulse of hormone secretion that begins or ends a developmental period, as occurs at several points during insect metamorphosis (Nijhout 1994, 1999; Nijhout et al. 2014). This property is consistent also with the progressive time-varying patterns of gene expression during embryonic development (Tomancak et al. 2002).
This feature also adds a mode of pattern evolution. Pattern evolution could typically occur due to changes in parameter value reaction rates and gradient shapes. But it is also possible that evolutionary changes in the time when a developing pattern is frozen can lead to changes in the final color pattern. This adds a flexible mode of heterochromic evolution.
Moreover, if, as suggested above, the fixation of pattern depends on the timing of hormone secretion, this mechanism could also account for seasonal polyphenisms of butterfly color patterns. Seasonal polyphenisms in color patterns come about through changes in the timing of ecdysone secretion (Rountree and Nijhout 1995; Brakefield et al. 1998; Koch et al. 1996; Koch and Bückmann 1987) and thus may fix the progression of pattern at different stages. On this view, seasonally polyphenic patterns can be thought of as an expression of plastic heterochrony. Once a plastic pattern switch is established, additional adaptive changes in the patterning system can evolve to refine or further alter the pattern.
References
Baxter SW, Papa R, Chamberlain N, Humphray SJ, Joron M, Morrison C, Ffrench-Constant RH, Mcmillan WO, Jiggins CD (2008) Convergent evolution in the genetic basis of Mullerian mimicry in Heliconius butterflies. Genetics 180:1567–1577
Brakefield P, Gates J, Keys D, Kesbeke F, Wijngaarden P, Monteiro A, French V, Carroll S (1996) Development, plasticity and evolution of butterfly eyespot patterns. Nature 384:236–242
Brakefield P, Kesbeke F, Koch P (1998) The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. Am Nat 152:853–860
Brunetti CR, Selegue JE, Monteiro A, French V, Brakefield PM, Carroll SB (2001) The generation and diversification of butterfly eyespot color patterns. Curr Biol 11:1578–1585
Carroll S, Gates J, Keys D, Paddock S, Panganiban G, Selegue J, Williams J (1994) Pattern formation and eyespot determination in butterfly wings. Science 265:109–114
Crews SM, Mccleery WT, Hutson MS (2016) Pathway to a phenocopy: heat stress effects in early embryogenesis. Dev Dyn 245:402–413
Dhungel B, Otaki JM (2009) Local pharmacological effects of tungstate on the color-pattern determination of butterfly wings: a possible relationship between the eyespot and parafocal element. Zool Sci 26:758–764
Joron M, Jiggins CD, Papanicolaou A, Mcmillan WO (2006) Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity. Heredity 97:157–167
Kapan DD, Flanagan NS, Tobler A, Papa R, Reed RD, Gonzalez JA, Restrepo MR, Martinez L, Maldonado K, Ritschoff C, Heckel DG, Mcmillan WO (2006) Localization of Mullerian mimicry genes on a dense linkage map of Heliconius erato. Genetics 173:735–757
Koch P, Bückmann D (1987) Hormonal control of seasonal morphs by the timing of ecdysteroid release in Araschnia levana L. (Nymphalidae: Lepidoptera). J Insect Physiol 33:823–829
Koch PB, Nijhout HF (2002) The role of wing veins in colour pattern development in the butterfly Papilio xuthus (Lepidoptera: Papilionidae). Eur J Entomol 99:67–72
Koch P, Brakefield P, Kesbeke F (1996) Ecdysteroids control eyespot size and wing color pattern in the polyphenic butterfly Bicyclus anynana (Lepidoptera: Satyridae). J Insect Physiol 43:223–230
Kondo S, Miura T (2010) Reaction–diffusion model as a framework for understanding biological pattern formation. Science 329:1616–1620
Martin A, Reed RD (2014) Wnt signaling underlies evolution and development of the butterfly wing pattern symmetry systems. Dev Biol 395:367–378
Meinhardt H (1982) Models of Biological pattern formation. Academic, London
Mitchell H, Lipps L (1978) Heat shock and phenocopy induction in Drosophila. Cell Adhes Commun 15:907–918
Monteiro A (2015) Origin, development, and evolution of butterfly eyespots. Annu Rev Entomol 60:253–271
Monteiro A, Brakefield PM, Vernon F (1997) Butterfly eyespots: the genetics and development of the color rings. Evolution 51:1207–1216
Monteiro A, Prijs J, Bax M, Hakkaart T, Brakefield PM (2003) Mutants highlight the modular control of butterfly eyespot patterns. Evol Dev 5:180–187
Nadeau NJ (2016) Genes controlling mimetic colour pattern variation in butterflies. Curr Opin Insect Sci 17:24–31
Nijhout HF (1980) Pattern formation on lepidopteran wings: determination of an eyespot. Dev Biol 80:267–274
Nijhout HF (1985) Cautery induced colour patterns in Precis coenia (Lepidoptera: Nymphalidae). J Embryol Exp Morphol 86:191–203
Nijhout HF (1990) A comprehensive model for colour pattern formation in butterflies. Proc R Soc Lond B Biol Sci 239:81–113
Nijhout HF (1991) The development and evolution of butterfly wing patterns. Smithsonian Institution Press, Washngton, DC
Nijhout HF (1994) Insect hormones. Princeton, Princeton University Press
Nijhout HF (1999) Control mechanisms of polyphenic development in insects. Bioscience 49:181–192
Nijhout HF (2002) The nature of robustness in development. BioEssays 24:553–563
Nijhout HF (2010) Molecular and physiological basis of colour pattern formation. In: Jérôme C, Stephen JS (eds) Advances in insect physiology. Academic, London
Nijhout HF, Grunert LW (1988) Colour pattern regulation after surgery on the wing disks of Precis coenia (Lepidoptera: Nymphalidae). Development 102:337–385
Nijhout HF, Riddiford LM, Mirth C, Shingleton AW, Suzuki Y, Callier V (2014) The developmental control of size in insects. Wiley Interdiscip Rev Dev Biol 3:113–134
Otaki JM (2008) Phenotypic plasticity of wing color patterns revealed by temperature and chemical applications in a nymphalid butterfly Vanessa indica. J Therm Biol 33(2):128–139
Otaki JM (2009) Color-pattern analysis of parafocal elements in butterfly wings. Entomol Sci 12:74–83
Otaki JM (2011) Generation of butterfly wing eyespot patterns: a model for morphological determination of eyespot and parafocal element. Zool Sci 28:817–827
Reed RD, Serfas MS (2004) Butterfly wing pattern evolution is associated with changes in a notch/distal-less temporal pattern formation process. Curr Biol 14:1159–1166
Reed RD, Papa R, Martin A, Hines HM, Counterman BA, Pardo-Diaz C, Jiggins CD, Chamberlain NL, Kronforst MR, Chen R, Halder G, Nijhout HF, Mcmillan WO (2011) optix Drives the repeated convergent evolution of butterfly wing pattern mimicry. Science 333:1137–1141
Rountree DB, Nijhout HF (1995) Hormonal control of a seasonal polyphenism in Precis coenia (Lepidoptera: Nymphalidae). J Insect Physiol 41:987–992
Schwanwitsch BN (1924) On the ground-plan of wing-pattern in Nymphalids and certain other families of the Rhopaloeerous Lepidoptera. Proc Zool Soc Lond 94:509–528
Schwanwitsch BN (1929) Two schemes of the wing-pattern of butterflies. Z Morphol Okol Tiere 14:36–58
Süffert F (1929) Die Ausbildung der imaginalen Flügelschnittes in der Schmetterlingspuppe. Z Morphol Okol Tiere 14:338–359
Tomancak P, Beaton A, Weiszmann R, Kwan E, Shu S, Lewis SE, Richards S, Ashburner M, Hartenstein V, Celniker SE, Rubin GM 2002 Systematic determination of patterns of gene expression during Drosophila embryogenesis. Genome Biol 3, research0088.1-88.14.
Turing AM (1952) The chemical basis of morphogenesis. Philos Trans R Soc Lond Ser B Biol Sci 237:37–72
Welte MA, Duncan I, Lindquist S (1995) The basis for a heat-induced developmental defect: defining crucial lesions. Genes Dev 9:2240–2250
Zhang L, Reed RD (2016) Genome editing in butterflies reveals that spalt promotes and distal-less represses eyespot colour patterns. Nat Commun 7:11769
Acknowledgments
This work was supported by grants IOS-0641144, IOS-1121065, and IOS-155734 from the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Nijhout, H.F. (2017). The Common Developmental Origin of Eyespots and Parafocal Elements and a New Model Mechanism for Color Pattern Formation. In: Sekimura, T., Nijhout, H. (eds) Diversity and Evolution of Butterfly Wing Patterns. Springer, Singapore. https://doi.org/10.1007/978-981-10-4956-9_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-4956-9_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4955-2
Online ISBN: 978-981-10-4956-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)