Abstract
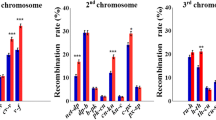
A highly inbred line of Drosophila melanogaster was subdivided into replicate sublines that were subsequently maintained independently with 10 pairs of parents per generation. The parents were randomly sampled for 19 ‘unselected’ sublines, and artificially selected for high or low abdominal or sternopleural bristle number for 12‘ selected’ sublines (with 3 replicate selection lines/trait/direction of selection). Divergence in mean bristle number among the unselected sublines, and response of the selected sublines to selection, are attributable to the accumulation of new mutations affecting bristle number. The input of mutational variance per generation, V M , can be estimated from the magnitude of response or divergence, assuming neutrality of mutations affecting the bristle traits. We reared unselected lines at generations 222 and 224, and selected lines at generations 182–184 of mutation accumulation at each of three temperatures (18 °C, 25 °C, 28 °C), and estimated the mutational variance common to all environments and the mutational variance from genotype × environment interaction. For sternopleural bristle number, the mutational interaction variance was 26% of the mutational variance common to all temperatures, and the interaction variance was due to temperature × line interaction. For abdominal bristle number, the mutational interaction variance was 142% of the mutational variance common to all temperatures, and the interaction variance was due to interactions of temperature × line, sex × line, and temperature × sex × line. It is possible that segregating variation for bristle number is maintained partly by genotype × environment interaction, but information on the fitness profiles of mutations affecting bristle number in each environment will be necessary to evaluate this hypothesis quantitatively.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Barton, N.H., 1990. Pleiotropic models of quantitative variation. Genetics 124: 773–782.
Caballero, A. & P.D. Keightley, 1994. A pleiotropic nonadditive model of variation in quantitative traits. Genetics 138: 883–900.
Clayton, G.A., J.A. Morris & A. Robertson, 1957. An experimental check on quantitative genetical theory. I. Short-term responses to selection. J. Genet. 55: 131–151.
Clayton, G.A. & A. Robertson, 1955. Mutation and quantitative variation. Am. Nat. 89: 151–158.
Cockerham, C.C., 1963. Estimation of genetic variances, pp. 53–94 in Statistical Genetics and Plant Breeding, edited by W.D. Hanson & H.F. Robertson. Washington, D.C.: National Academy of Sciences-National Research Council.
Crow, J.F. & M.J. Simmons, 1983. The mutation load in Drosophila, pp. 1–35 in The Genetics and Biology of Drosophila, edited by H.L. Carson, M. Ashburner & J.N. Thompson. London: Academic Press.
Durrant, A. & K. Mather, 1954. Heritable variation in a long inbred line of Drosophila. Genetica 27: 97–119.
Endler, J.A., 1986. Natural Selection in the Wild. Princeton, New Jersey: Princeton University Press.
Falconer, D.S., 1960. Selection of mice for growth on high and low planes of nutrition. Genet. Res. 1: 91–113.
Falconer, D.S., 1990. Selection in different environments: effects on environmental sensitivity (reaction norm) and on mean performance. Genet. Res. 56: 57–70.
Falconer, D.S. & T.F.C. Mackay, 1996. Introduction to Quantitative Genetics, 4/e. Harlow, Essex: Addison Wesley Longman.
Fernandez, J. & C. López-Fanjul, 1996. Spontaneous mutational variances and covariances for fitness-related traits in Drosophila melanogaster. Genetics 143: 829–837.
Fry, J.D., K.A. De Ronde & T.F.C. Mackay, 1995. Polygenic muta tion in Drosophila melanogaster: Genetic analysis of selection lines. Genetics 139: 1293–1307.
Fry, J.D., S.L. Heinsohn & T.F.C. Mackay, 1996. The contribution of new mutations to genotype-environment interaction for fitness in Drosophila melanogaster. Evolution 50: 2316–2327.
Garcia-Dorado, A. & J.A. Gonzalez, 1996. Stabilizing selection detected for bristle number in Drosophila melanogaster. evolution 50: 1573–1578.
Gillespie, J.H. & M. Turelli, 1989. Genotype-environment interac tion and the maintenance of polygenic variation. Genetics 121: 129–138.
Houle, D., B. Morikawa & M. Lynch, 1996. Comparing mutational evolvabilities. Genetics 143: 1467–1483.
Hill, W.G., 1982. Predictions of response to artificial selection from new mutations. Genet. Res. 40: 255–278.
Kearsey, M.J. & B.W. Barnes, 1970. Variation for metrical characters in Drosophila populations. II. Natural selection. Heredity 25: 11–21.
Keightley, P.D., 1996. Nature of deleterious mutation load in Drosophila. Genetics 144: 1993–1999.
Keightley, P.D. & A. Caballero, 1997. Genomic mutation rates for lifetime reproductive output and lifespan of C elegans. Proc. Natl. Acad. Sci. USA 94: 3823–3827.
Keightley, P.D. & W.G. Hill, 1990. Variation maintained in quantitative traits with mutation-selection balance: pleiotropic sideeffects on fitness traits. Proc. Roy. Soc. Lond. B. 242: 95–100.
Keightley, P.D., T.F.C. Mackay & A. Caballero, 1993. Accounting for bias in estimates of the rate of polygenic variation. Proc. Roy. Soc. Lond. B. 253: 291–296.
Kidwell, M.G., 1979. Hybrid dysgenesis in Drosophila melanogaster: the relationship between the P-M and I-R interac tion systems. Genet. Res. 33: 105–117.
Kondrashov, A.S. & M. Turelli, 1992. Deleterious mutations, appar ent stabilizing selection and the maintenance of quantitative vari ation. Genetics 132: 603–618.
Lande, R., 1975. The maintenance of genetic variability by mutation in a polygenic character with linked loci. Genet. Res. 26: 221–235.
Latter, B.D.H. & A. Robertson, 1962. The effects of inbreeding and artificial selection on reproductive fitness. Genet. Res. 3: 110–138.
Lai, C., R.F. Lyman, A.D. Long, C.H. Langley & T.F.C. Mackay, 1994. Naturally occurring variation in bristle number and DNA polymorphisms at the scabrous locus in Drosophila melanogaster. Science 266: 1697–1702.
Linney, R., B.W. Barnes & M.J. Kearsey, 1971. Variation for metrical characters in Drosophila populations. III. The nature of selection. Heredity 27: 163–174.
Long, A. D., S.L. Mullaney, T.F.C. Mackay & C.H. Langley, 1996. Genetic interactions between naturally occurring alleles at quan titative trait loci and mutant alleles at candidate loci affecting bristle number in Drosophila melanogaster. Genetics 144: 1497–1518.
Long, A. D., S.L. Mullaney, L.A. Reid, J.D. Fry, C.H. Langley & T.F.C. Mackay, 1995. High resolution mapping of genetic factors affecting abdominal bristle number in Drosophila melanogaster. Genetics 139: 1273–1291.
López, M.A. & C. López-Fanjul, 1993. Spontaneous mutation for a quantitative trait in Drosophila melanogaster. II. Distribution of mutant effects on the trait and fitness. Genet. Res. 61: 117–126.
Lyman, R.F., F. Lawrence, S.V. Nuzhdin & T.F.C. Mackay, 1996. Effects of single P element insertions on bristle number and via bility in Drosophila melanogaster. Genetics 143: 277–292.
Lynch, M. & W.G. Hill, 1986. Phenotypic evolution by neutral mutation. Evolution 40: 915–935.
Mackay, T.F.C., 1995. The genetic basis of quantitative variation: numbers of sensory bristles of Drosophila melanogaster as a model system. Trends Genet. 11: 464–470.
Mackay, T.F.C. & J.D. Fry, 1996. Polygenic mutation in Drosophila melanogaster: Genetic interactions between selection lines and candidate quantitative trait loci. Genetics 144: 671–688.
Mackay, T.F.C., J.D. Fry, R.F. Lyman & S.V. Nuzhdin, 1994. Polygenic mutation in Drosophila melanogaster: Estimates from response to selection of inbred strains. Genetics 136: 937–951.
Mackay, T.F. C. & C.H. Langley, 1990. Molecular and phenotypic variation in the achaete-scute region of Drosophila melanogaster. Nature 348: 64–66.
Mackay, T.F.C., R.F. Lyman & W.G. Hill, 1995. Polygenic muta tion in Drosophila melanogster: Non-linear divergence among unselected strains. Genetics 139: 849–859.
Mackay, T.F.C., R.F. Lyman & M.S. Jackson, 1992a. Effects of P ele ment insertions on quantitative traits in Drosophila melanogaster. Genetics 130: 315–332.
Mackay, T.F.C., R.F. Lyman, M.S. Jackson, C. Terzian & W.G. Hill, 1992b. Polygenic mutation in Drosophila melanogaster: Estimates from divergence among inbred strains. Evolution 46: 300–316.
Mukai, T., 1964. The genetic structure of natural populations of Drosophila melanogaster. I. Spontaneous mutation rate of polygenes controlling viability. Genetics 50: 1–19.
Mukai, T., S.I. Chigusa, L.E. Mettler & J.F. Crow, 1972. Mutation rate and dominance of genes affecting viability in Drosophila melanogaster. Genetics 72: 333–355.
Nuzhdin, S.V., J.D. Fry & T.F.C. Mackay, 1995. Polygenic mutation in Drosophila melanogaster: The causal relationship of bristle number to fitness. Genetics 139: 861–872.
Nuzhdin, S.V. & T.F.C. Mackay, 1994. Direct determination of retrotransposon transposition rates in Drosophila melanogaster. Genet. Res. 63: 139–144.
Nuzhdin, S.V. & T.F.C. Mackay, 1995. The genomic rate of transposable element movement in Drosophila melanogaster. Mol. Biol. Evol. 12: 180–181.
Ohnishi, O., 1977. Spontaneous and ethyl methanesulfonate-induced mutations controlling viability in Drosophila melanogaster: Homozygous effect of polygenic mutations. Genetics 87: 529–545.
Robertson, A., 1959. The sampling variance of the genetic correla tion coefficient. Biometrics 15: 469–485.
SAS Institute, Inc., 1988. SAS/STAT User’s Guide, Release 6.03 Edition. Cary, North Carolina: SAS Institute Inc.
Slatkin, M., 1987. Heritable variation and heterozygosity under a balance between mutations and stabilizing selection. Genet. Res. 50: 53–62.
Turelli, M., 1984. Heritable genetic variation via mutation-selection balance: Lerch’s zeta meets the abdominal bristle. Theor. Popul. Biol. 25: 138–193.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1998 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Mackay, T.F.C., Lyman, R.F. (1998). Polygenic mutation in Drosophila melanogaster: genotype × environment interaction for spontaneous mutations affecting bristle number. In: Woodruff, R.C., Thompson, J.N. (eds) Mutation and Evolution. Contemporary Issues in Genetics and Evolution, vol 7. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-5210-5_17
Download citation
DOI: https://doi.org/10.1007/978-94-011-5210-5_17
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-6193-3
Online ISBN: 978-94-011-5210-5
eBook Packages: Springer Book Archive