Abstract
Nuclear medicine studies of the central nervous system provide unique information on cerebral perfusion and metabolism and the physiology of cerebro-spinal fluid (CSF) fluid. Diagnosis of brain death is based on clinical findings indicating a complete lack of function of the entire brain. Mandatory additional studies supporting the clinical suspicion are required by most countries, such as electroencephalography (EEG), brainstem evoked response audiometry (BERA), transcranial Doppler, and brain scintigraphy. Brain scans following the administration of perfusion agents are considered to represent the most objective assessment tool. FDG PET and possibly ictal-interictal cerebral perfusion SPECT studies are used to identify an epileptogenic focus in drug-resistant epilepsy. The main indication is in patients with likely unifocal seizures at semiology and EEG, and a negative brain MRI, or with MRI findings not corresponding to clinical signs and symptoms and to EEG findings. CSF shunt patency studies involve injection of a radiopharmaceutical into the reservoir of a ventriculo-peritoneal shunt in children with hydrocephalus. The study shows the transit of labelled CSF through the shunt and may detect shunt obstructions. Radionuclide molecular imaging studies on brain tumors are rare and mainly part of research projects and are not performed in routine clinical settings and therefore will not be dealt with in this section.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Brain Death Study
2.1.1 Clinical Indications
-
Confirmation of brain death.
2.1.2 Pre-exam Information
-
Is there a strong clinical suspicion of brain death?
-
Did the patient sustain any recent injury to the head? If so, what is the location and severity of the injury?
-
Does the patient have intracranial pressure monitors or drip sites on the scalp?
-
What is the hemodynamic condition of the patient?
Study Protocol for Brain Death Imaging [1, 2]
Patient Preparation
-
In some institutions, when a non-specific radiopharmaceutical is used, a tourniquet is placed that encircles the head just above the eyebrows, ears, and around the posterior prominence of the skull. This reduces blood flow to the scalp and helps distinguish between cerebral and scalp activities.
Radiopharmaceutical, Administered Activity and Mode of Delivery
Radiopharmaceutical:
-
Preferred: specific brain perfusion tracers [99mTc]hexamethylpropyleneamine oxime (HMPAO) and [99mTc]ethyl cysteinate dimer (ECD) have the advantage that allows for the evaluation of the posterior fossa.
-
Alternative: brain non-specific perfusion tracers: [99mTc]Pertechnetate (Pertechnetate) or [99mTc]diethylene-triamine-pentaacetate (DTPA) have the advantage of low cost and availability.
Activity:
-
Weight based, 370–740 MBq (10–20 mCi) or 18 MBq/Kg (0.5 mCi/kg).
Refer to the EANM pediatric dosage card and to the North American consensus guidelines on radiopharmaceutical administration in children in the respective EANM and Society of Nuclear Medicine and Molecular Imaging (SNMMI) and image gently web sites.
Note the slight discrepancy: for HMPAO and ECD, the EANM dosage card indicates a minimum dose of 100 MBq.
Reference to national regulation guidelines, if available, should be considered.
Acquisition Protocol
-
Collimator: parallel-hole, low energy, high resolution.
-
Position: supine.
Using a brain-specific perfusion tracer
-
Dynamic study: anterior and posterior projections, 2 s/frame for a total of 2 min, matrix 128 x 128.
-
Static images: start immediately after dynamic images; anterior, posterior, and two lateral planar views, duration of acquisition 300 s, matrix 256 × 256.
-
SPECT: performed at 20–30 min after tracer injection, 25 s/step, 64 steps/head (for dual-head cameras), matrix 128 × 128.
-
SPECT/CT is recommended in cases of skull and scalp trauma or deformities.
Using a non-specific cerebral perfusion tracer
-
Dynamic study: anterior and posterior, 2 s/frame for a total of 2 min, matrix 128 × 128.
-
Static images: starting immediately after dynamic images; anterior, posterior, and two lateral views, duration of acquisition 300 s, matrix 256 × 256.
2.1.3 Study Interpretation [3]
-
Dynamic images are assessed while adjusting the window settings. Activity in the distribution of the anterior, middle, and posterior cerebral arteries and sagittal and lateral sinuses is evaluated.
-
The study should be assessed for the presence or absence of:
-
Cerebral perfusion on dynamic images.
-
Filling of the cerebral sinuses on planar images.
-
Uptake of the tracer in cerebral structures on SPECT.
-
In cases of brain death:
-
Arterial phase dynamic flow images show tracer distribution in the carotid arteries up to the base of the skull and no arterial blush over the cerebral hemispheres.
-
Venous phase dynamic flow images show no activity in the venous sinuses although faint activity might be noted on late images.
-
Static images confirm the absence of activity in the venous sinuses.
-
SPECT images show the total absence of activity in any part of the brain.
2.1.4 Correlative Imaging
-
Correlate with radiography for skull fracture.
-
CT and MRI may show evidence of anoxic brain injury with/without uncal herniation.
2.1.5 Red Flags
-
Use a line that is working well and try to give a good compact bolus.
-
Central lines are often used for administration of vasoactive agents and electrolytes. Consult with the referring physician or nurse if it is safe to inject a bolus into that line.
-
A peripheral line should be only used for tracer administration when a central line is unavailable.
-
Do not inject through an intravenous (IV) line placed in the scalp.
-
A tourniquet should not be placed prior to injection of non-specific tracers in cases of skull and scalp injuries.
-
Make sure that the entire head is in the field-of-view (FOV). Use a marker to confirm before giving the injection.
-
Static images must be performed immediately after the dynamic images. Diffusion of activity from the scalp through the epiploic veins into necrotic brain tissue and cerebral sinuses may occur.
-
If the patient is hemodynamically unstable the SPECT may be performed earlier than the 20–30 min specified in the study protocol.
-
If there is a history of traumatic brain injury with skull fractures and swelling, the study may be difficult to interpret due to soft tissue accumulation of Pertechnetate. Using a brain-specific perfusion tracer and SPECT/CT will improve diagnostic certainty.
-
Even the slightest tracer activity on SPECT in any part of the brain precludes the diagnosis of brain death. If noted, a repeat brain death scan should be performed within a few days. Most repeat scans will show disappearance of the residual tracer activity.
2.1.6 Take Home Messages
-
The final impression of the study should state that either there is no evidence or that it shows evidence of brain perfusion.
-
Brain-specific perfusion tracers can benefit from a SPECT or SPECT/CT acquisition which should be carefully evaluated.
-
Careful inspection of SPECT images should ensure that there is no tracer uptake in cerebral structures, in particular, infratentorial activity.
-
Brain death evaluation with non-specific tracers should only be used if brain-specific tracers are unavailable. DTPA is preferable to Pertechnetate.
-
SPECT/CT can distinguish uptake in the brain from increased activity related to hyperemia in overlying structures.
2.1.7 Representative Case Examples
Case 2.1. Brain Death (Fig. 2.1)
History: A 9-month-old boy admitted to the hospital with hypovolemic shock due to gastroenteritis had a cardiopulmonary arrest 2 days before the study was requested. The brain death study was performed using Pertechnetate. Study report: There is no perfusion to the cerebrum on the dynamic images (a). There is no filling of the cerebral sinuses on the static images (b). Impression: The study shows a lack of perfusion to the entire brain confirming the clinical diagnosis of brain death
Case 2.2. Equivocal Brain Scan in a Patient with Suspected Brain Death (Figs. 2.2 and 2.3)
History: A 14-year-old boy with congenital heart disease underwent cardiac surgery and had a sudden postoperative cardiovascular collapse. His pupils were dilated. He lost all brain stem reflexes. Brain CT demonstrated massive infarction of the entire left hemisphere and the right frontal lobe. His clinical status suggested brain death. A brain scan was performed following administration of ECD. Study report: Anterior radionuclide angiography (a) showed tracer distribution in the carotid arteries up to the base of the skull but no arterial blush in the brain. Increased tracer uptake in the facial soft tissues was noted especially in the nasal area (“hot nose sign”). Early static images (b) showed no tracer localization in the brain parenchyma, but the posterior and lateral views showed uptake in the cerebellum, further confirmed on SPECT (c). Impression: Brain death could not be confirmed because of the residual perfusion visualized in the cerebellum. The patient’s neurological status did not change, and a repeat study was obtained 5 days later
History: Repeat brain scan in a 14-year-old boy with suspected brain death and an equivocal study performed 5 days before. Study report: Anterior dynamic study (a) showed no cerebral perfusion. SPECT (b) showed no uptake in the cerebrum or cerebellum. Impression: The study confirms the clinical diagnosis of brain death
2.2 Drug-Resistant Epilepsy—Ictal and Interictal Brain Perfusion Spect
2.2.1 Clinical Indications
-
To help localize the epileptogenic focus in children with drug-resistant epilepsy considered for surgical excision in patients who have a normal or inconclusive MRI or with discordant MRI findings as compared to the clinical and video-telemetry results.
-
There must be a good pre-test likelihood of unifocal seizures (or at least a dominant seizure).
-
The seizure should last long enough to be captured by the tracer injection (seizures lasting less than 20 s are unlikely to be captured with an ictal SPECT, although the epileptogenic focus can still show prominent tracer uptake for some time after seizure ends).
-
2.2.2 Pre-exam Information
-
The need for general anesthesia or sedation is evaluated for an individual patient.
-
If general anesthesia is required, a slot should be available for both the morning and the afternoon scanning sessions, as the time when the patient fits is unknown.
-
Some flexibility in the appointment bookings of the day is essential as the time when the child is going to fit is unknown and therefore the ictal scan may have to be slotted in between other pre-booked examinations.
Study Protocol for Brain Perfusion Imaging in Epilepsy [4,5,6,7]
Patient Preparation
-
Ensure that the child has been admitted to the telemetry ward for at least 1–2 days to carefully study the EEG features of the seizures with the view to identify the characteristic seizure that has to be captured with the ictal tracer injection.
-
Ensure that the neurologist looking after the child has decided whether it is necessary to reduce the dose of the anti-epileptic medications or to stop them altogether for one of 2 days prior to the ictal injection.
-
Continuous EEG monitoring should start at least 2 h before the tracer injection and continue up to 15 min after the injection, to make sure that no seizures have occurred during the period of time leading up to tracer injection.
-
If the child is going to be anesthetized for the interictal study, this will happen after the tracer injection.
Radiopharmaceuticals, Administered Activity, and Mode of Delivery
Radiopharmaceuticals
-
[99mTc]ECD (ECD) is the radiopharmaceutical of choice if there is no radiopharmacy on site since it is more stable than the alternative [99mTc] HMPAO.
-
[99mTc]HMPAO (HMPAO) can be safely used for the ictal perfusion SPECT if there is a radiopharmacy on site.
-
[18F]Fluorodeoxyglucose (FDG) is often used for interictal studies.
Activity
-
ECD and HMPAO: 7.4–11.1 MBq/kg (0.2–0.3 mCi/Kg), minimum 111–185 MBq (3–5 mCi).
-
There are some differences between the North American consensus recommendations and the EANM dosage card as highlighted in the Image Gently website.
-
The North American consensus recommends 11.1 MBq (0.3 mCi/Kg), minimum dose of 185 MBq (5 mCi), and maximum of 740 MBq (20 mCi).
-
The EANM recommends a minimum dose of 100 MBq (2.7 mCi).
-
-
FDG: 3.7 MBq/kg (0.10 mCi/kg), minimal dose: 14 MBq (0.37 mCi).
Refer to the EANM pediatric dosage card and to the North American consensus guidelines on radiopharmaceutical administration in children in the respective EANM and SNMMI and image gently web sites.
Reference to national regulation guidelines, if available, should be considered.
Mode of Delivery
For the ictal SPECT study
-
When the characteristic seizure arises, the tracer has to be injected as soon as possible (within seconds) after seizure onset.
-
After the seizure has come to an end, the nuclear medicine department must be notified as soon as possible, so that they can alert the anesthesiologists (if the scan requires general anesthesia) and prepare the gamma camera for the ictal SPECT scan.
For the interictal SPECT study
-
There should be at least a 48-h interval after the ictal scan.
-
The tracer should be injected in the Nuclear Medicine department in a quiet room with dim lights.
-
A cannula must be placed at least 15 min prior to tracer injection.
-
The child is instructed not to speak, read, or move for at least 5 min prior and up to 5 min after the injection.
-
Acquisition should start at least 45 min after the injection of ECD or 40–90 min after the injection of HMPAO.
Acquisition Protocol
Patient positioning:
-
Supine with head as far up into the headrest as possible and shoulders as low as possible.
-
This could be aided by getting the patient to lay on a sheet that is pulled down over the shoulders and secured with a Velcro strap and the use of sponges or rolled-up pillowcases slotted on either side between the patient’s head and the headrest.
-
The patient needs to be as high as possible in the headrest to enable visualization of the back of the brain. Put the chin toward the chest to ensure that the entire cerebellum is included in the scan.
Acquisition parameters for SPECT:
-
Collimator: low-energy, high or ultra-high resolution.
-
Fan-beam collimator, if available, is preferred over a parallel-hole collimator.
-
SPECT parameters:
-
For triple head camera: 120 steps, 40 steps/ head, 20–25 secs/step.
-
For dual-head camera: 120 steps, 60 steps/head, 30 secs/step.
-
Acquisition mode: step-and-shoot is more frequently used. Continuous mode acquisition may provide shorter scan times and improve patient comfort.
-
Matrix 128 × 128.
-
Zoom: the acquisition pixel size should be one-third to one-half of the expected resolution.
-
2.2.3 Study Interpretation
-
The clinical and EEG details must be known.
-
A previously performed MRI using specific sequences for epilepsy should be available for comparison.
-
The telemetry report of the SPECT seizure must be available.
-
It is critical to have knowledge of the following information and record the timing (hh:mm:ss) where appropriate:
-
The exact time of EEG and clinical onset of the seizure.
-
The exact time of the tracer injection: start of the injection, end of the injection, end of flushing of the tracer with saline.
-
The exact time the seizure ends.
-
At least the time between flushing of tracer and seizure end (should be >15 secs).
-
EEG features at the time of seizure onset and immediately afterward.
-
2.2.4 Correlative Imaging [8,9,10,11,12]
-
A previously performed MRI using specific sequences for epilepsy should be available for comparison and may show flair abnormality.
-
Correlation with arterial spin labelling (ASL) sequences may be helpful to increase confidence in PET interpretation.
2.2.4.1 Red Flags
-
It is of the utmost importance to administer the ictal tracer injection as early as possible after seizure onset. However, what seemed to be a seizure in its early onset may not evolve into a full seizure and, if the tracer has been injected too soon, it will capture an event that does not declare itself as a real seizure.
-
The seizure ideally should not generalize into a tonic-clonic seizure shortly after tracer injection. If it does, when the tracer reaches the brain the electrical discharge will have propagated, resulting in several non-specific areas of hyperperfusion and therefore a lower likelihood of identifying the epileptogenic focus as an area of predominant hyperperfusion.
-
If the syringe with HMPAO (with stabilizer) has been in the telemetry ward for over 3 h without having been injected, it will be significantly dissociated between free Pertechnetate and HMPAO, with high uptake of free Pertechnetate in the salivary glands and with less uptake of HMPAO in the brain, resulting in poor quality images. If the child did not have a seizure within 3 h of the delivery of the syringe with HMPAO to the telemetry ward, another syringe with the tracer in it should be prepared and sent to the ward, and the previous unused syringe discarded.
-
It is critical to have knowledge of the following pieces of information:
-
The time between the end of flushing and end of seizure: if this is shorter than 15 s the ictal SPECT may fail to capture the focus.
-
If the seizure lasts less than 15–20 s, it may be difficult to capture with an ictal SPECT injection, since it takes about 15 s for the tracer to reach the brain after flushing with saline.
-
If the EEG findings during/immediately after the seizure can lateralize and localize the seizure focus, identify the origin of the seizure and whether it generalized shortly after onset.
-
-
If a fan-beam collimator is available, make sure that the whole head (including the cerebellum) is within the FOV.
-
The ictal SPECT scan must be interpreted with the knowledge of the findings of an EEG performed before, during, and after the tracer injection. Ictal SPECT findings, considered in isolation, could be seriously misleading.
-
The interictal SPECT study should also be performed always and correlated with the ictal study.
-
If the patient is not under general anesthesia, communication with the patient via squeezing the hand of an accompanying person may enable them to perform the study without risk of head movement during the scan.
2.2.5 Take Home Messages
-
This test should be done in centers with a specialized epilepsy surgery program.
-
The entire epilepsy team should be on site when the ictal study is performed; the results should be discussed during the multidisciplinary epilepsy team meeting.
-
Medical personnel trained and authorized in injection of radiopharmaceuticals must be dedicated to the child on the day of the ictal SPECT scan (the injection of the radiotracer occurs on the ward in a dedicated EEG-video-telemetry room).
-
The epilepsy team may reduce or stop anti-epilepsy medications prior to the ictal SPECT to induce the onset of the characteristic seizure.
-
Within the telemetry ward, a single room for the child is necessary. This room becomes a controlled area once the radiopharmaceutical is brought in.
-
Excellent communication between the telemetry ward, the nuclear medicine department, and the anesthetists is essential to coordinate the start of the SPECT images acquisition.
-
An interictal SPECT scan in isolation is an insufficient test in the evaluation of a child with drug-resistant epilepsy. An FDG PET interictal study can be performed instead of an interictal SPECT study.
-
Co-registration of the ictal SPECT and the interictal SPECT with the MRI is state-of-the-art and increases the diagnostic accuracy of each modality in the search a much better of the epileptic focus. EEG monitoring during injection of FDG tracer is important to confirm an interictal state.
2.2.6 Representative Case Examples
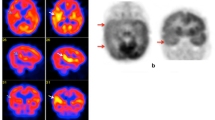
Case 2.3. Right Epileptogenic Focus (Fig. 2.4)
History: A 1-year-old developmentally delayed boy, with asymmetric infantile spasms and focal seizures refractory to anti-epileptic medications, had a negative MRI. EEG showed continuous abnormalities in the right hemisphere, possibly the right anterior quadrant. For the ictal SPECT, ECD was administered during a typical cluster of spasms, with EEG changes lasting approximately 6 min. The injection was given 2:39 min after clinical seizure onset. The seizures continued for 79 s after flushing of the tracer. The EEG at the time of injection showed an extensive area of abnormality suggestive of seizures from the right hemisphere, possibly right anterior quadrant. Study report: On the ictal study (upper row) there is increased tracer uptake in the right anterior frontal region. In the interictal study (lower row) performed 2 days later there is slightly reduced tracer uptake in the right anterior frontal region. Subtraction of interictal from ictal SPECT images (blue area upper row) shows a significant difference in radioactive counts in the anterior region of the right hemisphere, which correlates with EEG findings and clinical semiology. Impression: The findings suggest the presence of an epileptogenic focus in the anterior quadrant of the right hemisphere in the right frontal region. The patient was referred for invasive monitoring for further consideration of a possible right frontal resection
Case 2.4. Residual Right Epileptogenic Focus (Fig. 2.5)
History: A child who had a right posterior parietal resection for focal cortical dysplasia continued to present with seizures after surgery. Study report: An ictal ECD SPECT (a) shows highly increased tracer uptake in the right parietal region, adjacent to the resection margin. This same area shows reduced uptake in the interictal study (b). Impression: The findings suggest a residual epileptogenic focus after surgical resection. Review of MRI with specific sequences for epilepsy in this area showed changes compatible with residual focal cortical dysplasia. The child was referred for further surgery
Case 2.5. Right Focal Cortical Dysplasia (Fig. 2.6)
History: An 11-year-old boy had refractory partial and complex partial seizures, localized on EEG to the left frontal lobe. Study report: MRI showed a possible area of focal cortical dysplasia in the left frontal lobe (crosshairs MRI, left column). Ictal SPECT co-registered with MRI (center column) following injection of ECD shows focally increased tracer uptake in the left frontal area. This was confirmed in the interictal co-registered FDG PET/MRI study (right column). Impression: The study confirms an epileptogenic focus originating from the left focal frontal cortical dysplasia. Surgical resection was performed, and the patient is seizure-free at a 2-year follow-up
2.3 Cerebro-Spinal Shunt Patency Studies
2.3.1 Clinical Indications
-
Patients with hydrocephalus with a CSF shunt who have clinical symptoms such as headache and vomiting, or an imaging suspicion of a malfunctioning shunt.
Study Protocol for CSF Shunt Patency Imaging [13,14,15]
Patient Preparation
-
Review of medical history to determine the type of shunt (i.e., ventriculo-peritoneal, ventriculo-pleural, or lumbar-peritoneal).
-
Review of the patient’s CT and/or MRI to determine the site of the shunt reservoir.
-
Shave the hair over the shunt reservoir.
Radiopharmaceutical, Administered Activity and Mode of Delivery
Radiopharmaceutical:
-
[99mTc]DTPA (DTPA).
Activity:
-
20 MBq (0.55 mCi) in 0.1 ml volume drawn up in a tuberculin syringe.
Refer to the EANM pediatric dosage card and to the North American consensus guidelines on radiopharmaceutical administration in children in the respective EANM and SNMMI and image gently web sites. Reference to national regulation guidelines, if available, should be considered.
Mode of delivery:
-
Injected intrathecal or into the reservoir.
Acquisition Protocol
-
Collimator: parallel-hole, low energy, high resolution.
-
Position: supine.
-
FOV: Head and shunt pathway (chest or abdomen depending on shunt type).
-
Time of imaging: early, immediately, and at 40–90 min post-injection.
-
Static image acquisition parameters: 300 s/view or 100 Kcounts, matrix 256 × 256.
-
Posterior and lateral views of the head to detect reflux into the ventricles.
-
Anterior static images of the entire distal length of shunt tubing following the head images (generally chest and/or abdomen). If there is no dispersion of the tracer in the abdomen, the patient is encouraged to stand up and, if possible, walk around.
-
Additional, potentially required, delayed images up to 24 h of the head and chest/abdomen.
-
SPECT/CT, when available can be performed to better define the presence and localization of the radiotracer uptake.
-
For all images: note the time post-injection and label appropriately.
2.3.2 Study Interpretation
-
Assess the location and type of shunt reservoir.
-
Note whether CSF was readily withdrawn and sent for culture.
-
Record the presence of ventricular reflux and tube activity, the time when the tracer reached the peritoneal cavity/pleura, and if there was free tracer distribution within the abdomen/pleural cavity.
-
If free distribution of tracer is seen the distal end of the shunt is patent.
-
If no CSF was taken from the reservoir and there is slow transit down the tube and no free distribution of activity, the shunt is most likely blocked at the ventricular end.
2.3.3 Correlative Imaging
-
Radiography to confirm the type of shunt, i.e., ventriculo-atrial, ventriculo-pleural, or ventriculo-pelvic shunt.
-
Radiographs for detection of shunt tube disruption and position of end of tube.
2.3.4 Red Flags
-
A sterile technique should be used for drawing the dose and for the injection.
-
The technologist/ radiographer of the nuclear medicine department should be trained to safely prepare the radiopharmaceutical to be injected.
-
When performing intrathecal administration of radiopharmaceutical check for the purity of the compound and the activity to be injected.
-
A surgeon or a specifically trained nuclear medicine physician should administer the tracer injection according to national and hospital regulations.
2.3.5 Take Home Messages
-
The nurse/technologist assisting the doctor should press on the tube just below the injection site to induce reflux into the ventricles and thus test the patency of the proximal end.
-
A sample of CSF should be taken, if possible, for cytology and culture.
2.3.6 Representative Case Examples
Case 2.6. Patent Shunt (Fig. 2.7)
History: A 4-year-old boy with a ventriculo-peritoneal CSF diversion shunt for congenital hydrocephalus presents with a recent headache and vomiting. MR showed mild dilatation of the ventricles with no change from the previous study. A Rickham reservoir was initially accessed aseptically, and CSF fluid was withdrawn for culture. Study report: The tracer was injected into the Rickham reservoir (a, arrow) and passed rapidly from the ventricle into the shunt tube in the immediate post-injection images in posterior (a) and lateral (b) views. At 40 min post-injection (c) the tracer has distributed diffusely into the peritoneum. Impression: Patent ventriculo-peritoneal shunt
Case 2.7. Blocked Shunt (Fig. 2.8)
History: A 7-year-old boy with a history of hydrocephalus secondary to a brain tumor, which had been successfully removed followed by insertion of a ventriculo-peritoneal shunt, developed lethargy and headache with occasional vomiting. MRI showed moderate dilatation of the ventricles. Study report: The tracer was injected via a Hakim reservoir (a, red arrow). The CSF appeared under increased pressure. On the immediate post-injection image in lateral projection, the tracer flows back into the ventricle (a, blue arrow) indicating a patent proximal end. On images of the chest and abdomen performed 1 h post-injection (b) the tracer is shown to have passed slowly down the shunt tube and does not distribute into the peritoneal cavity. Mild renal excretion is seen. Impression: The findings indicate a blocked distal end of the ventriculo-peritoneal shunt
References
Donohoe KJ, et al. SNM practice guideline for brain death scintigraphy 2.0. J Nucl Med Technol. 2012;40(3):198–203.
American College of Radiology. ACR-ACNM-SNMMI-SPR Practice parameter for the performance of single-photon emission brain perfusion imaging (Including SPECT and SPECT/CT). 2021.
Zuckier LS, Kolano J. Radionuclide studies in the determination of brain death: criteria, concepts, and controversies. Semin Nucl Med. 2008;38(4):262–73.
Van Paesschen W, et al. The use of SPECT and PET in routine clinical practice in epilepsy. Curr Opin Neurol. 2007;20(2):194–202.
la Fougère C, et al. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009;15(1):50–5.
Juni JE, et al. Procedure guideline for brain perfusion SPECT using (99m)Tc radiopharmaceuticals 3.0. J Nucl Med Technol. 2009;37(3):191–5.
Kapucu OL, et al. EANM procedure guideline for brain perfusion SPECT using 99mTc-labelled radiopharmaceuticals, version 2. Eur J Nucl Med Mol Imaging. 2009;36(12):2093–102.
Patil S, Biassoni L, Borgwardt L. Nuclear medicine in pediatric neurology and neurosurgery: epilepsy and brain tumors. Semin Nucl Med. 2007;37(5):357–81.
Stanescu L, et al. FDG PET of the brain in pediatric patients: imaging spectrum with MR imaging correlation. Radiographics. 2013;33(5):1279–303.
Mountz JM, Patterson CM, Tamber MS. Pediatric epilepsy: neurology, functional imaging, and neurosurgery. Semin Nucl Med. 2017;47(2):170–87.
Tóth M, et al. The role of hybrid FDG-PET/MRI on decision-making in presurgical evaluation of drug-resistant epilepsy. BMC Neurol. 2021;21(1):363.
Khalaf AM, Nadel HR, Dahmoush HM. Simultaneously acquired MRI arterial spin-Labeling and interictal FDG-PET improves diagnosis of Pediatric temporal lobe epilepsy. AJNR Am J Neuroradiol. 2022;43(3):468–73.
Treves ST, et al. Central nervous system: the brain and cerebrospinal fluid. In: Treves ST, editor. Pediatric nuclear medicine and molecular imaging. Springer New York, New York, NY; 2014. p. 47–97.
Khalatbari H, Parisi MT. Complications of CSF shunts in Pediatrics: functional assessment with CSF shunt scintigraphy-performance and interpretation. AJR Am J Roentgenol. 2020;215(6):1474–89.
Kranz PG, et al. CSF-venous fistulas: anatomy and diagnostic imaging. AJR Am J Roentgenol. 2021;217(6):1418–29.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the IAEA: International Atomic Energy Agency, its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the IAEA: International Atomic Energy Agency, provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the IAEA: International Atomic Energy Agency that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the IAEA: International Atomic Energy Agency's name for any purpose other than for attribution, and the use of the IAEA: International Atomic Energy Agency's logo, shall be subject to a separate written license agreement between the IAEA: International Atomic Energy Agency and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Biassoni, L., Nadel, H., Bar-Sever, Z. (2023). Central Nervous System: The Brain and Cerebro-Spinal Fluid. In: Bar-Sever, Z., Giammarile, F., Israel, O., Nadel, H. (eds) A Practical Guide for Pediatric Nuclear Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-67631-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-662-67631-8_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-67630-1
Online ISBN: 978-3-662-67631-8
eBook Packages: MedicineMedicine (R0)