Abstract
Chemical mutagens, such as ethyl methanesulfonate (EMS) and sodium azide (NaN3), interact with DNA and can primarily induce single base modifications along the genome. Populations derived from chemical mutagenesis experiments are presumed to harbor high density of point mutations in the genome. Therefore, this technique, along with in vitro culture methods such as somatic embryogenesis (SE), can introduce genetic variation in otherwise genetically homogeneous populations. In vitro mutagenesis of embryogenic cell suspension cultures represents an efficient method to quickly develop mutant plantlets of unicellular origin. The development of mutant populations in this important crop represents a fundamental steppingstone in the development of novel varieties and the characterization of candidate genes involved in traits such as disease resistance, grain metabolite content and flowering induction. This chapter describes the protocol for establishment of embryogenic cell suspension cultures as well as methods of mutation induction using EMS and NaN3 on embryogenic cell suspensions of C. arabica, variety Catuaí. Furthermore, this chapter includes a protocol for mutant plant regeneration in in vitro conditions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The combination of chemical mutagenesis with in vitro culture techniques offers advantages to improve the efficiency of mutagenic treatments. The easier management of large populations of plants and the independence of agronomic and environmental factors can be listed among these advantages (Xu et al. 2011). In vitro selection procedures may also be applied to accelerate some screening steps and mutant lines can be quickly micropropagated. Success of these protocols depends on the establishment of robust in vitro regeneration procedures. It is advisable to apply mutagenic treatments on culture methods that involve regeneration via individual cells. This way, chimeric events could be avoided in most cases, or at least, can be dissolved more rapidly. Somatic embryogenesis (SE) is one of the ideal systems which could be incorporated in a mutation breeding program. It can be described as a morphogenic process characterized by the formation of embryos from somatic cells without fecundation (Campos et al. 2017). Somatic embryos or embryogenic calli usually are formed from a limited number of cells on the plant tissue, thus rendering a mostly unicellular origin for the regenerated plantlets. Chimeric events can be reduced when embryogenic cultures are mutagenized and time limitations in breeding programs can be overcome. The predominant unicellular origin of embryogenic structures facilities the early and direct screening of M1V1 plantlets regenerated from M1V1 treated calli or tissues without the need to develop an M2 generation (Serrat et al. 2014).
When using embryogenic cultures, variables such as survival of cells and regeneration capacity after mutagenic treatment must be assessed to optimize the mutagen dose(s). Dual tests that allow qualitative and quantitative viability analysis are advised. One of the most widely used assays for checking the viability of in vitro cultures is the 2,3,5-triphenyltetrazolium chloride (TTC) test used to differentiate between metabolically active and inactive tissues. In living tissues TTC is converted to a red colored precipitate 1,3,5-triphenylformazan (TPF) that can be easily detected and quantified. Somatic embryos regenerated from mutagenized tissues can show germination and growth delay, germination inhibition can also appear.
Coffee is a key driver in social development and cultural identity of many tropical and subtropical regions. Worldwide production of coffee relies on two species, Coffea arabica L. (60%) and C. canephora (40%). The better cup quality and higher market value are associated to C. arabica L., the only allotetraploid (2n = 4x = 44) species among Coffea. C. arabica is an autogamous plant mostly incompatible with the remainder of Coffea species (Anthony et al. 2002). These characteristics, along with severe bottlenecks that happened during coffee domestication led to reduced genetic variability in C. arabica populations; this reduction enhances the general susceptibility of many C. arabica L. genotypes to diseases (Hendre and Aggarwal 2007).
SE has been developed in coffee, both directly through proembryogenic cells and indirectly through embryogenic calli from leaf explants. Embryogenic cultures are induced on an auxin containing medium, thereafter, subculture on auxin free medium induces embryo regeneration (Gatica-Arias et al. 2008; Quiroz-Figueroa et al. 2002; van Boxtel and Berthouly 1996). Coffee SE has been widely studied and represents an useful tool in breeding (reviewed by Campos et al. 2017). In this chapter, we describe methods for the mutagenic treatment of in vitro coffee embryogenic cultures to induce genetic variability. The chapter covers the application of the chemical mutagens EMS and sodium azide on the C. arabica var. Catuaí embryogenic cell suspensions as well as the regeneration of mutant plantlets.
2 Materials
2.1 Plant Material
-
1.
Mature coffee cherries (e.g. Coffea arabica L. var. Catuaí) (see Note 1).
-
2.
Embryogenic cell suspension cultures (e.g. Coffea arabica L. var. Catuaí) (see Note 1).
2.2 Reagents
-
1.
10% (w/v) sodium thiosulfate (e.g. Sigma Cat Nr.: 217263).
-
2.
100 mM phosphate buffer (pH 3.0).
-
3.
1 N HCl (e.g. Phytotechnology Cat Nr.: H245).
-
4.
1 N KOH (e.g. Phytotechnology Cat Nr.: P682).
-
5.
1 N NaOH (e.g. Phytotechnology Cat Nr.: S835).
-
6.
2,3,5-triphenyltetrazolium (TTC) (e.g. Phytotechnology Cat Nr.: T8164).
-
7.
2,4-Dichlorophenoxyacetic acid (2,4-D) (e.g. Sigma Cat Nr.: D7299).
-
8.
3-Indoleacetic acid (IAA) (e.g. Sigma Cat Nr.: I2886).
-
9.
6-(γ,γ-dimethylallylamino)-purine (2-iP) (e.g. Sigma Cat Nr.: D7257).
-
10.
6-Benzylaminopurine (BAP) (e.g. Sigma Cat Nr.: D3408).
-
11.
Absolute ethanol.
-
12.
Adenine sulfate (e.g. Phytotechnology Cat Nr.: A545).
-
13.
Biotin (e.g. Phytotechnology Cat Nr.: B140).
-
14.
Calcium pantothenate (e.g. Phytotechnology Cat Nr.: C186).
-
15.
Casein hydrolysate (e.g. Phytotechnology Cat Nr.: C184).
-
16.
Citric acid (e.g. Phytotechnology Cat Nr.: C277).
-
17.
Ethyl methanesulphonate (EMS) (e.g. Sigma Cat Nr.: M0880).
-
18.
Gelling agent (e.g. Phytagel: Sigma Cat Nr.:P8169).
-
19.
Glycine (e.g. Phytotechnology Cat Nr.: G503).
-
20.
Phosphorus acid (H3PO3) (e.g. Sigma Cat Nr.: 176680).
-
21.
Indole-3-butyric acid (IBA) (e.g. Sigma Cat Nr.: I5386).
-
22.
KH2PO4 (e.g. Sigma Cat Nr.: P5655).
-
23.
Kinetin (e.g. Sigma Cat Nr.: K1885).
-
24.
l-cysteine (e.g. Phytotechnology Cat Nr.: C204).
-
25.
Malt extract (e.g. Phytotechnology Cat Nr.: M474).
-
26.
Murashige and Skoog (MS) basal salt mixture (e.g. Phytotechnology Cat Nr.: M524).
-
27.
Myo-inositol (e.g. Phytotechnology Cat Nr.: I703).
-
28.
Nicotinic acid (e.g. Phytotechnology Cat Nr.: N765).
-
29.
Phosphorus acid (H3PO3) (e.g. Sigma Cat Nr.: 176680).
-
30.
Pyridoxine HCl (e.g. Phytotechnology Cat Nr.: P866).
-
31.
Sodium azide (NaN3) (e.g. Sigma Cat Nr.: S2002).
-
32.
Sodium hypochlorite.
-
33.
Sterile distilled water.
-
34.
D-Sucrose (e.g. Phytotechnology Cat Nr.: S829).
-
35.
Thiamine HCl (e.g. Phytotechnology Cat Nr.: T390).
-
36.
Tissue culture grade water.
-
37.
Tween 20 (e.g. Phytotechnology Cat Nr.: P720).
2.3 Glassware and Minor Equipment
-
1.
0.22 µm millipore filter.
-
2.
Baby food jars (e.g. Phytotechnology Cat Nr.: C1770).
-
3.
Beakers (100 ml, 500 ml, and 1,000 ml).
-
4.
Bottles (100 ml, and 500 ml).
-
5.
Box for dry hazardous material disposal.
-
6.
Closures for culture tubes (e.g. Phytotechnology Cat Nr.: C1805).
-
7.
Culture tubes (25 mm × 150 mm).
-
8.
Disposable pipettes (1 ml, 5 ml, 10 ml, and 25 ml).
-
9.
Erlenmeyer flasks (250 ml).
-
10.
Forceps.
-
11.
Glass or disposable pipettes (1 ml, 5 ml, 10 ml, 25 ml).
-
12.
Graduated cylinder (50 ml, 100 ml, 500 ml and 1,000 ml).
-
13.
Hazardous liquid waste receptacle (collection vessels for NaN3 and EMS waste solution).
-
14.
Magnetic stir bar.
-
15.
Personal protective equipment (disposable laboratory coat dedicated only to mutagenesis experiments, eye protection/goggles, shoe protection, nitrile gloves).
-
16.
Petri dishes (100 mm × 20 mm).
-
17.
Pipette rubber bulb or electronic pipette controller.
-
18.
Reaction tubes (2 ml, 15 ml).
-
19.
Scalpels.
-
20.
Scalpel blades.
-
21.
Spatula.
-
22.
1 ml syringe.
-
23.
Volumetric flasks (50 ml, 100 ml, and 1,000 ml).
-
24.
Weighing trays.
2.4 Equipment
-
1.
Analytical balance.
-
2.
Autoclave.
-
3.
Centrifuge.
-
4.
Chemical mutagen laboratory equipped with fume hood and flow bench (see Notes 2 and 3).
-
5.
Hot plate shaker.
-
6.
Magnetic stir bar.
-
7.
Medium dispenser.
-
8.
Orbital shaker.
-
9.
pH meter.
-
10.
Spectrophotometer.
-
11.
Stereoscope.
-
12.
Water bath.
2.5 Tissue Culture Media
-
1.
Semi-solid development medium (DEV), pH 5.6.
-
2.
Semi-solid germination medium (EG), pH 5.6.
-
3.
Semi-solid regeneration medium (R), pH 5.6.
-
4.
TEX liquid culture medium, pH 5.6.
-
5.
TEX liquid culture medium (pH 3.0 and pH 5.6).
2.6 Software
-
1.
Standard spreadsheet software (e.g. Microsoft Excel or Open Office Excel).
3 Methods
3.1 Preparation of Stock Solutions
-
1.
100 mM Phosphate buffer: add 680.5 mg of KH2PO4 to 250 ml volumetric flask and dissolve by adding 25 ml of tissue culture grade water. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Adjust pH to 3.0 using phosphorus acid (H3PO3).
-
2.
2,4-Dichlorophenoxyacetic acid (2,4-D, 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH or 95% v/v ethanol. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at 4 °C.
-
3.
3-Indoleacetic acid (IAA; 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at − 20 °C.
-
4.
6-(γ,γ-dimethylallylamino)-purine (2-iP; 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at − 20 °C.
-
5.
6-Benzylaminopurine (BAP; 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at 4 °C.
-
6.
Indole-3-butyric acid (IBA; 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH or 95% (v/v) ethanol. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at 4 °C.
-
7.
KH2PO4 (0.07 M): add 907.8 mg of the powder to 250 ml volumetric flask and dissolve by adding 50 ml of tissue culture grade water. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 100 ml.
-
8.
Kinetin (KIN; 1 mg/ml stock solution): add 50 mg of the powder to a 100 ml volumetric flask and dissolve by adding 2–5 ml of 1 N NaOH. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store aliquots (1 ml) at − 20 °C.
-
9.
Na2HPO4 (0.08 M): add 1.1876 g of the powder to 250 ml volumetric flask and dissolve by adding 50 ml of tissue culture grade water. Once completely dissolved, stir the solution while adding tissue culture grade water and bring to 100 ml.
-
10.
Sodium azide (NaN3) (500 mM): add 1.625 g of the powder to 250 ml volumetric flask and dissolve by adding 50 ml phosphate buffer (100 mM). Once completely dissolved, stir the solution while adding phosphate buffer (100 mM) and bring to 50 ml. Sterilize by filtering through a 0.2 μm filter and store at 4 °C and protect it from light.
-
11.
2,3,5-triphenyltetrazolium (TTC) stock solution: add 4 ml of KH2PO4 (0.07 M) and 6 ml of Na2HPO4 (0.08 M). Adjust pH to 7. Add TTC to reach 1% (m/v) (100 mg for 10 ml of solution). Sterilize by filtering through a 0.2 μm filter and store aliquots in the dark at 4 °C.
3.2 Preparation of Tissue Culture Media
-
1.
Prepare stock solutions of IAA (1 mg/ml), 2,4-D (1 mg/ml), 2-iP (1 mg/ml), BAP (1 mg/ml), Biotin (1 mg/ml), Calcium pantothenate (1 mg/ml), Glycine (1 mg/ml), IBA (1 mg/ml), KIN (1 mg/ml), Nicotinic acid (1 mg/ml), Pyridoxine HCl (1 mg/ml), Thiamine HCl (1 mg/ml), adenine sulfate (10 mg/ml), l-cysteine (1 mg/ml).
-
2.
Place a beaker containing 400 ml tissue culture grade water on a hot plate shaker and mix:
-
(a)
For 1 L of callus induction medium: half-strength MS salts, 10 ml thiamine HCl, 1 ml pyridoxine HCl, 1 ml nicotinic acid, 1 ml glycine, 100 mg myo-inositol, 100 mg casein hydrolysate, 400 mg malt extract, 0.5 ml 2,4-D, 1 ml IBA, 2 ml 2-iP, 30 g sucrose, 2 g Phytagel™, pH 5.6.
-
(b)
For 1 L of embryo induction medium: half-strength MS salts, 20 ml thiamine HCl, 20 ml glycine, 40 ml l-cysteine, 200 mg myo-inositol, 6 ml adenine hemisulfate salt, 200 mg casein hydrolysate, 800 mg malt extract, 1 ml 2,4-D, 4 ml BAP, 30 g sucrose, 2 g Phytagel™, pH 5.6.
-
(c)
For 1 L of liquid proliferation medium (CP): half-strength MS salts, 5 ml thiamine HCl, 0.5 ml pyridoxine HCl, 0.5 ml nicotinic acid, 10 m l-cysteine, 50 mg myo-inositol, 100 mg casein hydrolysate, 200 mg malt extract, 2 ml 2,4-D, 1 ml KIN, 30 g sucrose, pH 5.6.
-
(d)
For 1 L of regeneration medium (R): half-strength MS salts, 10 ml thiamine HCl, 1 ml pyridoxine HCl, 1 ml nicotinic acid, 2 ml glycine, 200 ml myo-inositol, 4 ml adenine hemisulfate salt, 400 mg casein hydrolysate, 400 mg malt extract, 4 ml BAP, 40 g sucrose, 2.5 g Phytagel™, pH 5.6.
-
(e)
For 1 L of germination medium (EG): half-strength MS salts, 8 ml thiamine HCl, 3.2 ml pyridoxine HCl, 100 mg myo-inositol, 0.45 ml IAA, 0.25 ml BAP and 2.5 g Phytagel™, pH 5.6.
-
(f)
For 1 L of development medium (DEV): full-strength MS salts 1 ml thiamine HCl, 1 ml pyridoxine HCl, 1 ml nicotinic acid, 1 ml calcium pantothenate, 0.01 ml biotin, 100 mg myo-inositol, 0.3 ml BAP, 30 g sucrose, 2.5 g Phytagel™, pH 5.6.
-
(g)
For 1 L of TEX medium (Teixeira et al. 2004): half-strength MS salts, 10 ml thiamine HCl, 1 ml pyridoxine HCl, 1 ml glycine, 250 mg citric acid, 10 ml l-cysteine, 100 mg/L myo-inositol, 100 mg/L casein hydrolysate, 200 mg/L malt extract, 1 ml 2,4-D, 1 ml IBA, 2 ml 2-iP, 20 g sucrose, pH 5.6.
-
(a)
-
3.
While stirring, add tissue culture grade water to a final volume of 1,000 ml.
-
4.
Stir until the solution is homogenous and clear.
-
5.
Calibrate the pH meter as per manufacturer instructions.
-
6.
While stirring, adjust medium to pH 5.6 using 1 N NaOH or 1 N HCl.
-
7.
For semi-solid medium, add the gelling agent and heat while stirring until the solution is homogenous and clear.
-
8.
Dispense the culture medium in the respective culture vessel before or after autoclaving (depending on the application).
-
9.
Sterilize all media in a validated autoclave at 1 kg/cm2 for 21 min at 121 °C.
-
10.
Allow the medium to cool prior to use.
-
11.
Store the medium for up to a week in a cold room.
3.3 Germination of Coffee Zygotic Embryos Under In Vitro Conditions
-
1.
Collect mature cherries from genetically homogenous mother plants maintained in the greenhouse or in the field (see Note 4).
-
2.
Remove the pulp, the mucilage, and the parchment by hand.
-
3.
Soak the seeds for 24 h in distilled water with two drops of Tween 20 with orbital rotation.
-
4.
Disinfect the seeds with 3.5% (v/v) sodium hypochlorite for 1 h and finally rinse three times with sterile distilled water.
-
5.
Remove the endosperm over the embryo using a scalpel and forceps and extract the embryo levering it out from the root pole with the same scalpel blade.
-
6.
Culture the zygotic embryos in test tubes containing 20 ml of germination medium (GER) and place them in the dark at 27 ± 2 °C for 8 weeks.
-
7.
Transfer the in vitro plantlets developed from these embryos to baby food jars with 20 ml of the above medium under a 16 h light photoperiod at 27 ± 2 °C.
-
8.
Subculture the in vitro plantlets to fresh development medium (DEV) every 90 days.
3.4 Protocol for Plant Regeneration via Somatic Embryogenesis
This protocol involves a series of sequential stages: callus formation with embryogenic structures; establishment and multiplication of embryogenic suspension cultures; formation, maturation, and germination of somatic embryos; and conversion to plants; field evaluation (van Boxtel and Berthouly 1996) (see Fig. 1).
3.4.1 Embryogenic Callus Culture Initiation
-
1.
Take the first and second completely developed leaf from the in vitro plantlets and cut leaf sections measuring 0.5 cm2, without the midvein and the margins.
-
2.
Culture the leaf pieces with the abaxial surface upwards on 20 ml of callus induction medium (van Boxtel and Berthouly 1996) contained in baby food jars for 4 weeks in the dark at 27 ± 2 °C.
-
3.
Then, transfer the necrotic primary callus and explants to baby food jars containing 20 ml of the embryo induction medium (van Boxtel and Berthouly 1996).
-
4.
Incubate the baby food jars during 6–8 months under a 16 h low-light photoperiod (10 μE/m2/s) at 27 ± 2 °C until yellow or whitish embryogenic callus appears on the primary calli that have initially developed on the cut edges.
3.4.2 Establishment of Embryogenic Suspension Cultures
-
1.
Weigh 250 mg of friable embryogenic callus and transfer to 25 ml liquid proliferation medium CP (van Boxtel and Berthouly 1996) in 250 ml Erlenmeyer flasks.
-
2.
Incubate the Erlenmeyer flasks on a gyratory shaker at 100 rpm at 27 ± 2 °C in the dark.
-
3.
Every 15 days, replace the old medium with 50 ml fresh liquid proliferation medium.
3.4.3 Regeneration of Somatic Embryos and Development into Plantlets
-
1.
Culture 250 mg fresh weight of suspension cultures in Petri dishes (100 mm × 20 mm) containing 20 ml of regeneration medium (R) (van Boxtel and Berthouly 1996).
-
2.
Incubate the petri dishes under a 16 h light photoperiod at 27 ± 2 °C for 8–10 weeks.
-
3.
Transfer the somatic embryos to baby food jars containing 20 ml of germination medium (GER) with 16 h light photoperiod at 27 ± 2 °C for 6–8 weeks.
-
4.
Transfer the plantlets to baby food jars containing 20 ml development medium (DEV) with 16 h light photoperiod at 27 ± 2 °C.
3.4.4 Hardening of In Vitro Plantlets in the Greenhouse
-
1.
Do a transverse cut in the stem of the plants, below a node, and remove the leaves near the cut.
-
2.
Place the freshly cut basal part of the stem in a solution of indoleacetic acid (IAA) (1 mg/mL) for 30 s.
-
3.
Plant the seedlings in sterile substrate (peat moss with perlite) in plastic boxes (30 cm × 20 cm × 10 cm).
-
4.
Cover the boxes with plastic and place in a growth room with controlled conditions (25 °C, photoperiod of 12 h).
-
5.
Two weeks later, make small holes in the plastic covering of the boxes.
-
6.
After five weeks, evaluate the rooting percentage and transfer the plants with emerged roots to the greenhouse. Keep the rest of the plants under controlled growth conditions for up to 8 weeks.
-
7.
At the end of 8 weeks, determine the percentage of rooting and transfer those plants with roots to the greenhouse.
-
8.
In the greenhouse, place the plants in polyethylene bags with a 3:1 mixture of substrate (peat moss with perlite: coconut fiber). Plant two plants per bag and identify according to the original numbering.
-
9.
After 3 weeks, fertilize with granular slow-release fertilizer (e.g., Osmocote 14-14-14).
-
10.
Irrigate the plants twice a week according to the climatic conditions of the greenhouse and the water requirement of the crop.
3.5 Determination of the Viability of the Embryogenic Calli
-
1.
Weigh 100 mg of embryogenic calli sample.
-
2.
Place the sample in a 2 ml reaction tube and add 1 ml of the TTC stock solution.
-
3.
Incubate the samples for 24 h in the dark at 37 °C without shaking.
-
4.
Remove the TTC solution by decanting or centrifugation and wash the sample with distilled water.
-
5.
Add 1 ml of 95% (v/v) ethanol.
-
6.
Extract the formazan by placing the samples in a water bath at 65 °C for 10 min with constant shaking.
-
7.
Centrifuge the sample at 2,500 rpm and recover the supernatant.
-
8.
Quantify absorbance at 490 nm in a spectrophotometer.
3.6 Sodium Azide Dose Determination
-
1.
Review safety procedures of the chemical mutagenesis laboratory (see Notes 2 and 3).
-
2.
Autoclave all non-disposable materials (e.g. sieves, forceps).
-
3.
Prepare a fresh 500 mM NaN3 stock solution by adding the required amount of NaN3 to the phosphate buffer (100 mM, pH 3.0). Sterilize by filtering through a 0.2 μm filter using a sterile syringe.
-
4.
Discard the syringe and filter in the hazardous waste.
-
5.
Place 200 mg embryogenic calli in a 15 ml reaction tube with 10 ml of TEX medium (pH 3.0) (Teixeira et al. 2004).
-
6.
In a laminar flow cabinet, add appropriate concentrations of NaN3 solution. Shake the solution vigorously. Example dilutions and concentrations of NaN3 are given in Table 1.
-
7.
Label each tube with the appropriate treatment code (concentration and incubation time).
-
8.
Place closed tubes in the dark at 27 ± 2 °C on a rotary shaker set at 100 rpm for 15 min.
-
9.
After the incubation time, quickly but carefully decant each of the treatment batches and rinse the treated embryogenic suspension cultures with 10 ml of TEX medium (pH 5.6). This step and any subsequent steps must be carried out in a laminar flow cabinet.
-
10.
Repeat washing step 3 times.
-
11.
Collect all the liquid waste in a dedicated bucket labelled as hazardous waste. Dispose of toxic waste according to local regulations.
-
12.
Add 10 ml of TEX medium and maintain the cultures for 24 h in the dark with constant shaking at 100 rpm and 27 ± 2 °C.
-
13.
Record the absorbance and express the formazan content as a percentage of a positive control [(sample absorbance/absorbance of the control) × 100] as described in Sect. 3.5 and observe the cells using a stereoscope (see Note 5).
-
14.
Calculate the survival rate as follows: [(the number of survival explant/the number of treated explant) * 100].
-
15.
Alternatively, measure the absorbance values of the treated and non-treated embryogenic suspension cultures and determine survival rate.
-
16.
Record the data for each treatment and enter it into a spreadsheet (e.g., Microsoft Excel).
-
17.
Plot percentage of control against mutagenic treatment and estimate the mutagen concentration required to obtain 50% of viability compared to the control (LD50) (see Fig. 2) (see Note 7).
-
18.
Identify concentrations suitable for bulk mutagenesis of your material according to the LD50 previously estimated.
Effect of NaN3 concentration on survival and viability of coffee (C. arabica L. var. Catuaí) embryogenic calli. a Survival percentage (solid line) and absorbance (490 nm) (dotted line) versus NaN3 concentrations. Each value represents the mean ± SD of two repetitions, b cell viability versus NaN3 concentrations
3.7 Ethyl Methanesulphonate Dose Determination
-
1.
Review safety procedures of the chemical mutagenesis laboratory (see Notes 2 and 3).
-
2.
Autoclave all non-disposable materials (e.g. sieves, forceps).
-
3.
Sterilize EMS by filtering through a 0.2 μm filter using a sterile syringe and 2 ml collection tube.
-
4.
Discard the syringe, collection tube, and filter in the hazardous waste.
-
5.
Place 200 mg embryogenic calli in a 15 ml reaction tube with 10 ml of TEX medium (Teixeira et al. 2004).
-
6.
In a laminar flow cabinet, add appropriate concentrations of the EMS solution. Shake the solution vigorously. Example dilutions and concentrations for EMS are given in Table 2 (see Note 6).
-
7.
Label each tube with the appropriate treatment code (concentration and incubation time).
-
8.
Place closed tubes in the dark at 27 ± 2 °C on a rotary shaker set at 100 rpm and start incubation time.
-
9.
After the incubation time, quickly but carefully decant each of the treatment batches and rinse the treated embryogenic calli with 10 ml of TEX medium (pH 5.6). This step and any subsequent steps must be carried out in a laminar flow cabinet.
-
10.
Repeat washing step 3 times.
-
11.
Collect all the liquid waste in a dedicated bucket labelled as hazardous waste. Dispose of toxic waste according to local regulations.
-
12.
Add 10 ml of TEX medium and maintain the cultures for 24 h in the dark with constant shaking (100 rpm) at 27 ± 2°C.
-
13.
Determine cell viability of embryogenic calli and observe the cells using a stereoscope as described in Sect. 3.5 (see Note 5).
-
14.
Calculate the survival rate as follows: [(the number of survival explant/the number of treated explant) * 100]. Alternatively, measure the absorbance values of the treated and non-treated embryogenic suspension cultures and determine survival rate.
-
15.
Record the data for each treatment and enter it into a spreadsheet (e.g. Microsoft Excel).
-
16.
Plot percentage of control against mutagenic treatment and estimate the mutagen concentration required to obtain 50% of viability compared to the control (see Fig. 3) (see Note 7).
-
17.
Identify concentrations suitable for bulk mutagenesis of your material according to the LD50.
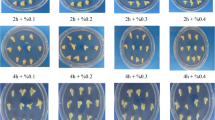
Effect of EMS concentration on survival and viability of coffee (C. arabica L. var. Catuaí) embryogenic calli. a Survival percentage (solid line) and absorbance (490 nm) (dotted line) after 60 min and 120 min of exposure time to different EMS concentrations. Each value represents the mean ± SD of two repetitions. b Cell viability versus EMS concentrations. Bar, 0.5 cm
3.8 Bulk Mutagenesis
-
1.
Autoclave all non-disposable materials (e.g. sieves, forceps).
-
2.
Choose appropriate NaN3 or EMS concentration and incubation time based on the results obtained from the chemical toxicity test.
-
3.
See Fig. 4 for an overview of the bulk mutagenesis procedure.
-
4.
Prepare embryogenic calli per each treatment chosen.
-
5.
Place 200 mg one-month-old embryogenic suspension cultures in a 15 ml reaction tube with 10 ml of TEX medium (Teixeira et al. 2004).
-
6.
Label each tube with NaN3 and EMS concentration and incubation time.
-
7.
Transfer closed tubes containing in vitro material into the chemical mutagenesis laboratory.
-
8.
In a laminar flow cabinet, add appropriate concentrations of NaN3 or EMS. Shake the solution vigorously.
-
9.
Place closed tubes in the dark at 27 ± 2 °C on a rotary shaker set at 100 rpm for the chosen length of time.
-
10.
After incubation, quickly but carefully decant each of the treatment batches and rinse the treated embryogenic suspension cultures with 10 ml of TEX medium (pH 5.6). This step and any subsequent steps must be carried out in a laminar flow cabinet.
-
11.
Repeat washing step 3 times.
-
12.
Collect all the liquid waste in a dedicated bucket labelled as hazardous waste. Dispose of toxic waste according to local regulations.
-
13.
After the final wash, add 10 ml of liquid regeneration medium (R) (pH 5.6) and maintain the cultures for 48 h in the dark on a rotary shaker (100 rpm) at 26 ± 2 °C.
-
14.
Carefully decant the treated embryogenic suspension cultures and plate them in Petri dishes containing 20 ml of R semisolid medium (see Note 8).
-
15.
Incubate the Petri dishes under a 16 h light photoperiod at 27 ± 2 °C for 8–10 weeks.
-
16.
Transfer the torpedo shape somatic embryos to baby food jars containing 20 ml of germination medium (GER) with 16 h light photoperiod at 27 ± 2 °C for 6–8 weeks (see Note 8).
-
17.
Cut off cotyledons and roots of the developed plantlets.
-
18.
Transfer the plantlets to baby food jars containing 20 ml of development medium (DEV) with 16 h light photoperiod at 27 ± 2 °C.
-
19.
Acclimatize plantlets with 4 leaves and 3–4 cm tall in the greenhouse as described in Sect. 3.4.4.
4 Notes
-
1.
This protocol has been established using Coffea arabica L. var. Catuaí seeds. It can be used as a reference for other Arabica coffee varieties, nevertheless, it is recommended to optimize the mutagenic parameter (NaN3 and EMS concentration and incubation time) for each variety used.
-
2.
All the mutagenesis experiments should be conducted in a dedicated chemical mutagenesis laboratory equipped with a ducted fume hood, toxic waste disposal and decontamination procedures.
-
3.
Read the Materials Safety Data Sheet (MSDS) of materials being used and follow the recommendation of the manufacturer. Pay careful attention to the information on sodium azide and EMS and what to do in case of exposure. It is very important to wear personal protective equipment (gloves must be compatible with chemical mutagens, for instance PVC or neoprene gloves); safety glasses with side shields or chemical goggles; lab coat, closed-toe shoes, shoe protections, and long trousers. A double glove system is advised.
-
4.
Coffee seeds loose viability rapidly if not properly stored. Therefore, it is recommended to use freshly harvested seeds or otherwise to store the seeds between 10 and 12% moisture at 15 °C for not longer than 3 months.
-
5.
Tetrazolium chloride staining has been used to evaluate the cell viability of embryogenic suspension cultures of grapevine treated with EMS (Acanda et al. 2014). In our study, there was a robust correlation between the survival percentage and the absorbance measured (NaN3, r2: 0.97; EMS, r2: 0.9) indicating that both methods could be used to determine cell viability.
-
6.
In our study, embryogenic suspension cultures treated with 5, 10, 15, 20, 80, 100, 120, and 140 mM EMS, did not show a reduction in viability of more than 15% compared to the non-treated embryogenic suspension cultures.
-
7.
Optimal dose determination of mutagenic treatment of in vitro cultures must be based on quantitative analysis of viability of cells exposed to the mutagen. When using embryogenic cultures, variables such as survival of cells and regeneration capacity after mutagenic treatment must be assessed to establish the dosage curve. Dual tests that allow qualitative and quantitative viability analysis are advised. One of the most widely used assays in in vitro cultures is the conversion of 2,3,5-triphenyltetrazolium (TTC) to its reduced form 1,3,5-triphenylformazan (TPF) in metabolically active (living) tissues. This process generates a red colored precipitate that can be easily detected and quantified.
-
8.
Somatic embryos regenerated from mutagenized tissues can show germination and growth delay, germination inhibition can also appear.
References
Acanda Y, Martínez O, Prado MJ, González MV, Rey M (2014) EMS mutagenesis and qPCR-HRM prescreening for point mutations in an embryogenic cell suspension of grapevine. Plant Cell Rep 33:471–481. https://doi.org/10.1007/s00299-013-1547-6
Anthony F, Combes MC, Astorga C, Bertrand B, Graziosi G, Lashermes P (2002) The origin of cultivated Coffea arabica L. varieties revealed by AFLP and SSR markers. Theor Appl Gent 104. http://doi.org/10.1007/s00122-001-0798-8
Campos NA, Panis B, Carpentier SC (2017) Somatic embryogenesis in coffee: the evolution of biotechnology and the integration of omics technologies offer great opportunities. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01460
Food and Agriculture Organization of the United Nations (2018) FAOSTAT database. FAO, Rome, Italy. Retrieved 23 Feb 2018 from www.fao.org/faostat/en/#search/coffee
Gatica-Arias AM, Arrieta G, Espinoza AM (2008) Plant regeneration via indirect somatic embryogenesis and optimisation of genetic transformation in Coffea arabica L. cvs. Caturra and Catuaí. Electron J Biotechnol 11:1–12
Hendre P, Aggarwal R (2007) DNA markers: development and application for genetic improvement of coffee. In: Varshney R, Tuberosa R (eds) Genomics-assisted crop improvement, vol 2. Springer, Dordrecht, NL, pp 399–434
Quiroz-Figueroa FR, Fuentes CFJ, Rojas R, Loyola V (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149
Serrat X, Esteban R, Guibourt N, Moysset L, Nogués S, Lalanne E (2014) EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods 10. https://doi.org/10.1186/1746-4811-10-5
Teixeira JB, Junqueira CS, Pereira JPC, Mello S, Silva PD, Mundim DA (2004) Multiplicação clonal de café (Coffea arabica L.) via embryogenesis somática. Embrapa Recursos Genéticos e Biotecnologia, Brasília (Embrapa Recursos Genéticos e Biotecnologia. Documentos, 121). Disponible en: http://www.cenargen.embrapa.br/publica/trabalhos/doc121.pdf
Van Boxtel J, Berthouly M (1996) High frequency somatic embryogenesis from coffee leaves: factors influencing embryogenesis, and subsequent proliferation and regeneration in liquid medium. Plant Cell Tissue Organ Cult 44(1):7–17
Xu C, Xiao J, He J, Hu G, Chen H (2011) The effect of ethyl methane sulphonate (EMS) and sodium azide (NaN3) on plant regeneration capacity of an embryogenic cell suspension of ‘Yueyoukang 1’ (Musa, aaa), a banana cultivar resistant to fusarium wilt. Acta Hortic 897:301–302. https://doi.org/10.17660/ActaHortic.2011.897.41
Acknowledgements
Funding for this work was provided by the University of Costa Rica, the Ministerio de Ciencia, Innovación, Tecnología y Telecomunicaciones (MICITT), the Consejo Nacional para Investigaciones Científicas y Tecnologicas (CONICIT) (project No. 111-B5-140; FI-030B-14) and the Cátedra Humboldt 2023. A. Gatica-Arias acknowledged the Cátedra Humboldt 2023 of the University of Costa Rica for supporting the dissemination of biotechnology for the conservation and sustainable use of biodiversity.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Gatica-Arias, A., Bolívar-González, A. (2023). Chemical Mutagenesis of Embryogenic Cell Suspensions of Coffea arabica L. var. Catuaí Using EMS and NaN3. In: Ingelbrecht, I.L., Silva, M.d.C.L.d., Jankowicz-Cieslak, J. (eds) Mutation Breeding in Coffee with Special Reference to Leaf Rust. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-67273-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-67273-0_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-67272-3
Online ISBN: 978-3-662-67273-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)