Abstract
Breeding heat tolerant rice is one of the strategies to develop crop adaptation to the effect of climate change, particularly in major rice growing regions that are vulnerable to increased temperature. Developing high temperature tolerant rice varieties is already an important breeding target for several national/international breeding programs; however, changing weather patterns have increased the urgency to develop heat stress tolerant rice varieties, particularly varieties that are already well adapted to local environments. Mutation breeding is an effective approach to develop heat stress tolerance in crops, including rice. Therefore, rice mutation breeding for adaptation to high temperature can augment current technology to maintain crop yields.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Heat Stress Tolerance
- Thermotolerance
- Plant Mutation Breeding
- Heat Shock Proteins (HSPs)
- Spikelet Fertility
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1.1 Background Analysis
Global warming has become a serious problem affecting agricultural production in vulnerable regions worldwide and is projected to worsen with anticipated climate change. Rice (Oryza sativa L.) is a major (staple) food crop associated with the lives of three billion people around the world. It is planted on about 159 million hectares annually in at least 114 countries by more than 100 million households in Asia and Africa (Tonini and Cabrera 2011). Rice is the source of 27% of dietary energy and 20% of dietary protein in the developing world and rice is the major staple crop for nearly half of the world’s population (Mottaleb et al. 2012).
Global environmental projections forecast that during the twenty-first century, global surface temperatures are likely to rise by 1.1 to 2.9 °C for the lowest carbon emission scenarios, and by 2.4 to 6.4 °C for the highest emission scenarios (IPCC 2012). The increase in temperature can cause irreversible damage to plant growth and performance, with major consequences on crop yield and also quality (Wahid et al. 2007). A 7–8% reduction in rice yield is associated with each 1 °C rise in day temperature from 28 to 34 °C (Baker et al. 1992). Using yield data from field experiments, Peng et al. reported that rice yields decline with higher night temperature from global warming. Increased temperatures cause reductions in the rate of photosynthesis and stomatal conductance at all growth stages in the life cycle of rice, at both vegetative and reproductive stages (Sanchez-Reinoso et al. 2014; Yoshida 1981).
Rice has been cultivated under a broad range of climatic conditions. Around 90% of the global rice crop is grown and consumed in Asia, where 50% of the population depends on rice as a regular and daily food (Pareek et al. 2010). In Asia the rice crop is particularly vulnerable to high temperatures (above 33 °C) during the sensitive flowering and early grain-filling stages (Wassmann et al. 2009a, b).
Regional high temperature damage to rice crops was also documented in many tropical and sub-tropical countries, such as Pakistan, India, Bangladesh, China, Thailand, Sudan and some other African countries (Osada et al. 1973; Matsushima et al. 1982; Li et al. 2004 ; Xia and Qi 2004; Yang et al. 2004; Tian et al. 2009). The problem is the most acute when temperature extremes coincide with critical sensitive stages in crop development. Heat tolerance for a crop is generally defined as the ability of plants to grow and produce an economic yield under high temperature (Wahid et al. 2007). Heat stress creates a serious threat to rice production, including in the most productive regions of the world, and it is imperative that heat tolerance is included as a target trait in breeding new rice cultivars (Pareek et al. 2010). In general, the reproductive stage is more vulnerable to heat stress than the vegetative stage in many crop species. In rice, almost all growth stages are affected by high temperature. During the vegetative growth period, rice can tolerate relatively high temperatures up to 35 °C. Temperatures above this level may reduce plant growth, flower initiation and ultimately yield. High temperatures are particularly damaging if they occur at the time of anthesis, and pollen shedding (Yoshida 1981). The two most sensitive stages are seedling stage, booting (microsporogenesis) and flowering (anthesis and fertilization). High temperature affects plant growth, meiosis, anther dehiscence, pollination, and pollen germination, which leads to spikelet sterility and yield loss (Yoshida 1981; Wassmann et al. 2009a, b; Shah et al. 2011; Prasanth et al. 2012; Tenorio et al. 2013; Sanchez-Reinoso et al. 2014). Exposure of rice plants to temperatures above 35 °C for short periods, less than one hour, during anthesis may result in varying degree of pollen and spikelet sterility which leads to significant yield losses and low grain quality (Jagadish et al. 2007; Matsui et al. 1997; Ye et al. 2015). Thus spikelet fertility under high temperature has been widely used as a screening index for heat tolerance at the flowering stage (Ye et al. 2015). Also the time of anthesis can affect sterility with early morning anthesis preferred since high temperature is avoided and hence high temperature induced sterility is reduced (Yoshida 1981).
Yield stability can only be improved if a breeding program is based on the valuable new knowledge on plant development and stress responses (Barnabas et al. 2008). A critical step in plant breeding is the ability to screen for the trait of interest. This study set out to develop simple, but effective protocols to screen for rare heat tolerant rice mutant plants among a host of non-improved siblings.
1.2 Physiology and Genetics of Heat Tolerance in Rice
Plant heat stress tolerance can be sub-divided into (1) escape; successful reproduction before the stress, such as the timing of panicle emergence and spikelet/floret opening before the occurrence of the stress (Singla et al. 1997), (2) avoidance; maintenance of a cooler canopy with higher transpiration from leaf surface, (3) true tolerance which may involve various physiological mechanisms induced under the stress (Kondamudi et al. 2012; Bahuguna et al. 2015; Bahuguna and Jagadish 2015). In rice a short exposure of seedlings to high temperature can affect the plant cellular ultrastructure with major changes occurring in the chloroplasts and mitochondria, thus resulting in reduced metabolism and hence also reduced growth (Pareek et al. 1997).
With respect to physiology, plants can adjust their metabolism and morphology in response to heat stress (Singla et al. 1997). High temperatures generally induce the expression of heat shock proteins (HSPs) and suppress, at least in part, the synthesis of normal cellular protein production (Shah et al. 2011). Heat shocks proteins (HSPs) are induced in response to short-term stress but may also be important to adapt to the heat stress (Pareek et al. 1995). HSPs can improve or stabilise photosynthesis, partitioning of assimilates, nutrient and water use efficiency and the thermal stability of cellular membranes (Wahid et al. 2007). Some of these HSPs and molecular chaperones aid in restoring damaged proteins (Kumari et al. 2013). These mechanisms need to be investigated further in agricultural production systems if they are to be exploited in developing heat stress-tolerant rice cultivars (Sailaja et al. 2015).
The genetics of heat tolerance is poorly understood, but is complex and controlled by multiple genes (Wahid et al. 2007; Xue et al. 2012; Driedonks et al. 2016). Heat tolerance in rice has a fairly high heritability and most genetic variation is additive (Yoshida 1981). There is huge variation for heat stress tolerance in rice as cultivars, lines and genotypes have been reported which are sensitive, tolerant or intermediate in response (see Ye et al. 2015). Many HSPs have been reported and their genetics (controlling genes, location of genes, dominance/recessive-ness) are known. However, certain gene combinations are critical to successful cultivar breeding, e.g. cultivars have to have the optimal genes/alleles for flowering time, height, etc., and it is not known how effective HSP genes are in an elite genetic background.
According to recent genetic studies plant heat-tolerance is probably a polygenic trait. In wheat different components of tolerance, controlled by different sets of genes, are critical for heat tolerance at different stages of development or in different tissues (Barakat et al. 2011). Shah et al. (2011) emphasized that indica rice is generally more heat tolerant than japonica rice, however there is a genotypic variation in spikelet fertility at high temperature in both species. Understanding the genetic basis of tolerance and enhancing the breeding level of heat tolerant cultivars in rice still continue. In rice, the development of molecular marker technology has led to the identification of several QTL for heat tolerance (Xue et al. 2012). It is known that the mapping populations and accurate phenotyping technology are essential for QTL mappings (Zhao et al. 2016). According to Zhong-Hua et al. (2014), after discovery of mutation through phenotyping, the mutant can be used for gene discovery. Thus far, 64 genes which are responsible for mutant phenotypes photosynthesis, signalling transduction and disease resistance have been isolated and mapped to the rice genome. Heat tolerance in rice at the flowering stage is controlled by several QTLs. Pyramiding validated QTL’s for heat tolerance QTL’s could be an important mechanism to enhance heat tolerance in rice at flowering stage, focused in spikelet fertility (Ye et al. 2015). They confirmed that the presence of recessive QTLs on chromosome 4 results in 15% higher fertile rice spikelet compared to plants without the QTL. Moreover Zhao et al. (2016) stated that using marker assisted selection (MAS) breeding strategy is essential, although many of putative QTLs for heat tolerance at anthesis have been identified, the effect and stability of the target QTL needs to be further confirmed.
The completion of the Rice Genome Sequencing Project and high-throughput genotyping and phenotyping have generated valuable data and tools that can be used to identify genes associated with target traits such as heat tolerance (Zhong-Hua et al. 2014). These advances will facilitate the dissection of genetic controls of heat tolerance in rice that may then be exploited in the development of new heat tolerant rice varieties.
1.3 Physiological and Biochemical Heat Stress Indicators in Rice
Heat stress alters a wide range of physiological, biochemical and molecular processes affecting crop growth and yield (Mittler et al. 2012; Hasanuzzaman et al. 2013). Photosynthesis is highly sensitive to heat stress, and above 35 °C decreases by 50% in rice. Another major physiological consequence of heat stress is augmented levels of reactive oxygen species in cells, which leads to oxidative stress (Hasanuzzaman et al. 2013). Plants can tolerate sub-lethal heat stress by avoidance, escape or physical changes at the cellular level such as changing membrane physical state, the synthesis of specialized HSPs and augmented anti-oxidative defence (Mittler et al. 2012; Bahuguna and Jagadish 2015). Plants acclimate to sub lethal heat stress by altering metabolism at physiological, biochemical and molecular levels. Changes in the membrane physical state and composition, production of heat shock proteins, transcription factors, osmolytes and augmented levels of antioxidant defence are key processes to maintain cellular redox homeostasis under heat stress (Krasensky and Jonak 2012). Heat stress alters gene expression patterns (Shinozaki and Yamaguchi-Shinozaki 2007) leading to the acclimation and/or adaptation to heat stress with improved antioxidant defence and higher levels of heat shock proteins, which can protect the integrity of proteins and other biological molecules (Moreno and Orellana 2011). Moreover, plants can modify their metabolism in various ways in response to heat stress, notably by generating compatible solutes that are able to organize proteins and cellular structures, maintain cell turgor pressure, and modify anti-oxidant mechanisms to re-establish cellular redox homeostasis (Munns and Tester 2008; Janska et al. 2010). Heat stress also alters gene expression which involves ‘direct protection’ from high temperature stress (Shinozaki and Yamaguchi-Shinozaki 2007). These proteins include osmo-protectants, transporters, anti-oxidants and regulatory proteins (Krasensky and Jonak 2012). Moreno and Orellana (2011) indicated that in heat stress, alterations in physiological and biochemical processes caused by gene expression progressively lead to the development of heat tolerance in the form of acclimation and/or adaptation, but this may not be associated with yield.
Key physiological and biochemical indicators of heat stress tolerance include electrolyte leakage, lipid peroxidation level i.e. malondialdehyde (MDA) content and anti-oxidant enzyme activity (Campos et al. 2003; Heath and Packer 1986; Bajji et al. 2001; Nakano and Asada 1981; Oberley and Spitz 1985). High temperatures may also affect membrane stability through lipid peroxidation, leading to the production of peroxide ions and MDA. A change in concentration of MDA is a good indicator of membrane structural integrity under temperature stress (Sanchez-Reinoso et al. 2014). Increase temperature stress 37 °C/30 °C (day/night) increased MDA content and electro leakage percentage in rice (Zhang et al. 2009; Liu et al. 2013).
1.4 Breeding for Heat Tolerance in Rice
Induced mutation has been hugely successful in rice breeding and could augment ongoing breeding efforts for enhancing heat stress tolerance in rice and other crops (Forster et al. 2014). Thus far, 824 rice cultivars have been developed by mutation breeding using mostly gamma irradiation, but also Ethyl methane sulfonate (EMS) and fast neutron (IAEA 2017 mutant variety database). One significant example is the first semi-semi-dwarf dwarf rice cultivar, Calrose 76, developed with 15% higher yield than taller cultivars, and it has also been used as a source of many semi-dwarf dwarf cultivars by rice scientists (Zhong-Hua et al. 2014). Another significant example is Zhefu 802 mutant rice variety was grown in China 10.6 million ha from 1986 to 1994 in China (Shu et al. 1997).
Although heat-tolerant rice genotypes have been found in both sub-species (Prasad et al. 2006) it was noted that indica spp. are more tolerant to higher temperatures than japonica spp. (Satake and Yoshida 1978). Recently, new heat stress tolerant rice cultivars have been generated by conventional cross breeding; examples include heat-tolerant lines and released cultivars such as NH 219, Dular, Nipponpare and WAB56-125. These are popular heat tolerant cultivars in South East Asia, particularly in the Philippines, Vietnam, Thailand, Indonesia and Cambodia (Poli et al. 2013; Manigbas et al. 2014). Moreover high quality Pon-Lai rice developed through cross breeding with a parent japonica type good quality mutant variety and another parent indica type heat stress tolerant variety to breed heat tolerant and high quality rice in Taiwan (Wu et al. 2016). In addition five heat stress tolerant japonica cultivars were bred from 2005 to 2011 in Japan (Takahashi et al. 2016).
To date, the indica rice genotype N22, an EMS induced mutant which is a deep-rooted, is the most tolerant genotype for heat stress and drought (Yoshida et al. 1981; Prasad et al. 2006; Poli et al. 2013). Many studies have demonstrated genotypic variation in spikelet sterility at high temperatures (Satake and Yoshida 1978; Prasad et al. 2006) and the fertility of spikelets at high temperature can be used as a screening tool for reproductive stage (Shah et al. 2011). A drawback of conventional breeding is that the programmes are often based on local elite lines with low genetic diversity (Driedonks et al. 2016) and consequently unlikely to possess variation for new traits such as heat tolerance. Wide crossing with more exotic material may provide the required genetic variation, but the breeding process will take longer to clean up the genetic background after the initial cross.
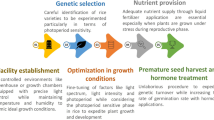
Induced mutation is a heritable change in the genetic material of living organisms, and this has been a major driver in species diversity and evolution. Plant breeding requires genetic variation of useful traits for crop improvement. The use of various mutagens to generate genetic variation in crop plants has a history almost as long as that of conventional breeding. Mutation breeding involves the development of new cultivars by generating new genetic variability induced by chemical and physical mutagens. Once mutation is produced the next steps are to detect and identify mutants with desired traits, i.e. screening. Mutant selection is a key step in a mutation breeding program, requiring the screening of thousands of mutants to recover the rare mutant with the desired trait. Hence, a major bottleneck in plant mutation breeding is effective screening of rare desired mutants having genetically improved characteristics among a mutant population comprising thousands of plants.
A major challenge in breeding for heat tolerance is the identification of reliable screening methods and effective selection criteria to facilitate detection of heat-tolerant plants. Several screening methods and selection criteria have been developed by different researchers (Wahid et al. 2007; Ye et al. 2015), but for practical plant breeding these need to be rapid and efficient in terms of time, space and cost. Due to the complexity of heat stress, there is need to develop quick and fast screening protocols for heat tolerance and plant breeders are still in need for identifying such efficient screening tools for detecting heat tolerance potentials at early growth stages in crops. Therefore, there is an urgent need for reliable pre-field screening protocols to enhance the efficiency and effectiveness of plant mutation breeding.
Heat stress alters a wide range of physiological, biochemical and molecular processes affecting crop growth and yield (Mittler et al. 2012; Hasanuzzaman et al. 2013). Photosynthesis is highly sensitive to heat stress, and above 35 °C decreases by 50% in rice. Another major physiological consequence of heat stress is augmented levels of reactive oxygen species in cells, which leads to oxidative stress (Hasanuzzaman et al. 2013). Plants can tolerate sub-lethal heat stress by avoidance, escape or physical changes at the cellular level such as changing membrane physical state, the synthesis of specialized HSPs and augmented anti-oxidative defence (Mittler et al. 2012; Bahuguna and Jagadish 2015). Plants acclimate to sub lethal heat stress by altering metabolism at physiological, biochemical and molecular levels. Changes in the membrane physical state and composition, production of heat shock proteins, transcription factors, osmolytes and augmented levels of antioxidant defence are key processes to maintain cellular redox homeostasis under heat stress (Krasensky and Jonak 2012). Heat stress alters gene expression patterns (Shinozaki and Yamaguchi-Shinozaki 2007) leading to the acclimation and/or adaptation to heat stress with improved antioxidant defence and higher levels of heat shock proteins, which can protect the integrity of proteins and other biological molecules (Moreno and Orellana 2011). Moreover, plants can modify their metabolism in various ways in response to heat stress, notably by generating compatible solutes that are able to organize proteins and cellular structures, maintain cell turgor pressure, and modify anti-oxidant mechanisms to re-establish cellular redox homeostasis (Munns and Tester 2008; Janska et al. 2010). Heat stress also alters gene expression which involves ‘direct protection’ from high temperature stress (Shinozaki and Yamaguchi-Shinozaki 2007). These proteins include osmo-protectants, transporters, anti-oxidants and regulatory proteins (Krasensky and Jonak 2012). Moreno and Orellana (2011) indicated that in heat stress, alterations in physiological and biochemical processes caused by gene expression progressively lead to the development of heat tolerance in the form of acclimation and/or adaptation, but this may not be associated with yield.
Key physiological and biochemical indicators of heat stress tolerance include electrolyte leakage, lipid peroxidation level i.e. Malondialdehyde (MDA) content and anti-oxidant enzyme activity (Campos et al. 2003; Heath and Packer 1986; Bajji et al. 2001; Nakano and Asada 1981; Oberley and Spitz 1985). High temperatures may also affect membrane stability through lipid peroxidation, leading to the production of peroxide ions and MDA. A change in concentration of MDA is a good indicator of membrane structural integrity under temperature stress (Sanchez-Reinoso et al. 2014). Increase temperature stress 37 °C/30 °C (day/night) increased MDA content and electro leakage percentage in rice (Zhang et al. 2009; Liu et al. 2013).
References
Bahuguna RN, Jagadish KS (2015) Temperature regulation of plant phenological development. Environ Exp Bot 111:83–90
Bahuguna RN, Pal JJ, Shah D, Lawas LM, Khetarpal S, Jagadish KS (2015) Physiological and biochemical characterization of NERICA rice: a novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol Plant 154(4):543–559
Bajji M, Kinet JM, Lutts S (2001) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 00:1–10
Baker JT, Allen LH, Boote KJ (1992) Temperature effects on rice at elevated CO2 concentration. J Exp Bot 43:959–964
Barakat MM, Al-Doss AA, Elshafei AA, Moustafa KA (2011) Identification of new microsatellite marker linked to the grain filling rate as indicator for heat tolerance genes in F2 wheat population. AJCS 5(2):104–110. ISSN:1835-2707
Barnabas B, Jager K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. Plants. J Plant Physiol 160:283–292
Driedonks N, Rieu I, Vrizen WH (2016) Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod 29:67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4909801/
Forster BP, Till BJ, Ghanim AMA, Huynh HO, Burstmayr H, Caligari PD (2014) Accelerated plant breeding. CAB Rev 43:1–16
Hasanuzzaman M, Nahar K, Fujita M (2013) Extreme temperatures, oxidative stress and antioxidant defense in plants. In: Vahdati K, Leslie C (eds) Abiotic stress-plant responses and applications in agriculture. Tech Rijeka, Croatia, pp 169–205
Heath RL, Packer L (1986) Photoperoxidarion in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
IAEA (2017) Mutant variety database. https://nucleus.iaea.org/Pages/mvd.aspx
IPCC (2012) Managing the risks of extreme events and disasters to advance climate change adaptation. https://www.ipcc.ch/pdf/special-reports/srex/SREX_Full_Report.pdf
Jagadish SVK, Craufurd PQ, Wheler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.) J Exp Bot 58:1627–1635
Janska A, Marsik P, Zelenkova S, Ovesna J (2010) Cold stress and acclimation: what is important for metabolic adjustment. Plant Biol 12:395–405
Kondamudi R, Swamy KN, Chakravarthy DVN, Vishnuprasanth V, Rao YV, Rao PR, Sarla N, Subrahmanyam D, Voleti SR (2012) Heat stress in rice – physiological mechanisms and adaptation strategies page under the book crop stress and its management perspective and strategies. In: Venkateswarlu B, Shanker AK, Maheswari M (eds) Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 193–223
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63(4):1593–1608. https://doi.org/10.1093/jxb/err460
Kumari S, Roy S, Singh P, Singla-Pareek SL, Pareek A (2013) Cyclophilins: proteins in search of function. Plant Signal Behav 8:e22734
Li CY, Peng CH, Zhao QB, Xie P, Chen W (2004) Characteristic analysis of the abnormal high temperature in 2003 midsummer in Wuhan City. J Cent Chin Norm Univ 38:379–381
Liu QH, Wu X, Li T, Ma JQ, Zhou XB (2013) Effects of elevated air temperature on physiological characteristics of flag leaves and grain yield in rice. Chil J Agric Res 73:85–89
Manigbas N, Lambio LA, Madrid LB, Cardenas CC (2014) Germplasm innovation of heat tolerance rice for irrigated conditions in the Philippines. Rice Sci 21:162–169
Matsui T, Omasa K, Horie T (1997) High temperature induced spikelet sterility of japonica rice at flowering in relation to air humidity and wind velocity conditions. Jpn J Crop Sci 66:449–455
Matsushima S, Ikewada H, Maeda A, Honda S, Niki H (1982) Studies on rice cultivation in the tropics, yielding and ripening responses of the rice plant to the extremely hot and dry climate in Sudan. Jpn J Trop Agric 26:19–25
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37:118–125
Moreno AA, Orellana A (2011) The physiological role of the unfolded protein response in plants. Biol Res 44:75–80
Mottaleb KA, Rejesus RM, Mohanty S, Murty MVR, Li T, Valera HG, Gumma MK (2012) Ex ante assessment of a combined drought- and submergence-tolerant rice variety in the presence of climate change. Paper presented at the Agricultural & Applied Economics Association annual meeting, Seattle, Washington, USA, 12–14 August, IRRI, Philipinnes
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oberley LW, Spitz DR (1985) Assay of superoxide dismutase using nitroblue tetrazolium. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, FL, pp 217–227
Osada A, Sasiprapa V, Rahong M, Dhammanuvong S, Chakrabandho H (1973) Abnormal occurrence of empty grains of indica rice plants in the dry hot season in Thailand. Proc Crop Sci Soc Jpn 42:103–109
Pareek A, Singla SL, Grover A (1995) Immunological evidence for accumulation of two high molecular weight (104 and 90 kDa) HSPs in response to different stresses in rice and in response to high temperature stress in diverse plant genera. Plant Mol Biol 29:293–301
Pareek A, Singla SL, Grover A (1997) Short-term salinity and high temperature stress-associated ultrastructural alterations in young leaf cells of Oryza sativa L. Ann Bot 80:629–639
Pareek A, Sopory SK, Bohnert H, Govindjee J (2010) Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Springer, Dordrecht, ISBN: 978-90-481-3111-2
Poli Y, Basava RK, Panigrahy M, Vinukonda VP, Dokula NR, Voleti SR (2013) Characterization of a Nagina22 rice mutant for heat tolerance and mapping of yield traits. Rice 6:36. https://doi.org/10.1186/1939-8433-6-36
Prasad PVV, Bootee KJ, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop Res 95:398–411
Prasanth VV, Chakravarthi DVN, Vishnu KT, Venkateswara RY, Panigrahy M, Mangrauthia SK (2012) Evaluation of rice germplasm and introgression lines for heat tolerance. Ann Biol Res 3:5060–5068
Sailaja BD, Subrahmanyam S, Neelamraju T, Vishnukiran YV, Rao P, Vijalakshmi SR, Voleti VP, Mangrauthia K (2015) Integrated physiological, biochemical, and molecular analysis identifies important traits and mechanisms associated with differential response of rice genotypes to elevated temperature. Front Plant Sci 6:1044–1055
Sanchez-Reinoso AD, Garces-Varon G, Restrepo-Diaz H (2014) Biochemical and physiological characterization of three rice cultivars under different daytime temperature conditions. Chil J Agric Res 74(4). Chillán dic. 2014
Satake T, Yoshida S (1978) High temperature induced sterility in indica rice at flowering. Jpn J Crop Sci 47:6–17
Shah F, Huang J, Kul K, Nie L, Shah T, Chen C (2011) Impact of high-temperature stress on rice plant and its traits related to tolerance. J Agric Sci 149:545–556. https://doi.org/10.1017/S0021859611000360
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Shu QY, Wu DX, Xia Y (1997) The most widely cultivated rice variety ‘Zhefu 802’ in China and its geneology. Mut Breed Newsl 43:3–5
Singla SL, Pareek A, Grover A (1997) High temperature in rice. In: Prasad MNV (ed) Plant ecophysiology. Wiley, New York, pp 101–127
Takahashi S, Miyazaki M, Matsuo K, Suriyasak C, Tarnada A, Matsuyama K, Iwaya-Inoque M, Ishibashi Y (2016) Differential responses to high temperature during maturation in heat-stress-tolerant cultivars of Japonica rice. Plant Prod Sci 19:2016–2012
Tenorio FA, Ye C, Redona E, Sierra S, Laza M, Argayoso MA (2013) Screening rice genetic resources for heat tolerance. SABRAO J Breed Genet 45:371–381
Tian X, Luo H, Zhou H, Wu C (2009) Research on heat stress of rice in China: progress and prospect. Chin Agric Sci Bull 25:166–168
Tonini A, Cabrera E (2011) Globalizing rice research for a changing world (Technical Bulletin No. 15). International Rice Research Institute, Los Banos
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–233
Wassmann R, Jagadish SVK, Sumfleth K, Pathak H, Howell G, Ismail A, Serraj R, Redoña E, Singh RK, Heuer S (2009a) Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv Agron 102:93–105
Wassmann R, Jagadish SVK, Heuer S, Ismail A, Redona E, Serraj R (2009b) Climate change affecting rice production: the physiological and agronomic basis for possible adaptation strategies. In: Sparks DL (ed) Advances in agronomy, vol 101. Academic Press, Burlington, pp 59–122
Wu Y, Chang SJ, Lui HS (2016) Effects of high temperature on grain yield and quality of a subtropical japonica rice- Pon Lai rice. Plant Prod Sci 19(1):145–153
Xia MY, Qi HX (2004) Effects of high temperature on the seed setting percent of hybrid rice bred with four male sterile lines. Hubei Agric Sci 2:21–22
Xue DW, Jiang H, Hu J, Zhang XQ, Guo LB, Zeng DL, Dong GJ, Sun GC, Qian Q (2012) Characterization of physiological response and identification of associated genes under heat stress in rice seedlings. Plant Physiol Biochem 61(2012 Dec):46–53
Yang HC, Huang ZO, Jiang ZY, Wang XW (2004) High temperature damage and its protective technologies of early and middle season rice in Anhui province. Anhui Agric Sci 32:3–4
Ye C, Tenerio FA, Argayoso MA, Laza MA, Koh HJ, Redeno ED, Jagadish SVK, Gregorio GB (2015) Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet 16:41. https://bmcgenet.biomedcentral.com/articles/10.1186/s12863-015-0199-7
Yoshida S (1981) Fundamentals of rice crops science. Los Banos, The Philippines IRRI
Yoshida, S., Satake T, Mackill DS (1981) High temperature stress in rice (review). IRRI Res. Paper Series 67
Zhang GL, Chen LY, Zhang ST, Zheng H, Liu GH (2009) Effects of high temperature stress on microscopic and ultrastructural characteristics of mesophyll cells in flag leaves of rice. Ric Sci 16:65–71
Zhao L, Lei J, Huang Y, Zhu S, Chen H, Huang R, Peng Z, Tu Q, Shen X, Yan S (2016) Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed Sci 66(3):358–366
Zhong-hua W, Xin-chen, Yu-lin J (2014) Development and characterization of rice mutants for functional genomic studies and breeding. Rice Sci:307–332, http://www.wageningenacademic.com/doi/10.3920/978-90-8686-787-5_16
Author information
Authors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (https://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the IAEA, provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the IAEA that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the IAEA's name for any purpose other than for attribution, and the use of the IAEA's logo, shall be subject to a separate written license agreement between the IAEA and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 International Atomic Energy Agency
About this chapter
Cite this chapter
Sarsu, F. (2018). General Introduction. In: Pre-Field Screening Protocols for Heat-Tolerant Mutants in Rice. Springer, Cham. https://doi.org/10.1007/978-3-319-77338-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-77338-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-77337-7
Online ISBN: 978-3-319-77338-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)