Abstract
Synthetic polymers are one of the most significant pollutants in the aquatic environment. Most research focused on small plastic particles, so-called microplastics (particle size, 1–5,000 μm). Compared to macroplastics, the small size complicates their determination in environmental samples and demands for more sophisticated analytical approaches. The detection methods of microplastics reported in the past are highly diverse. This chapter summarizes different strategies for the sampling of water and sediment and sample treatments, including the separation of plastic particles and removal of natural debris that are necessary prior the identification of microplastics. Moreover, the techniques used for the identification of plastics particles are presented in this chapter.
With the application of the method described in this chapter, microplastics were detected in freshwater systems, such as rivers and lakes worldwide. The abundance of microplastics reported in the studies varied in more than three orders of magnitude.
Furthermore, microplastics are not uniform, as there are many different types of synthetic polymers commercially available. Consequently, a variety of different polymer types is present in the aquatic environment. The knowledge on the type of polymer provides additional information for scientists: the type of polymer dictates its physicochemical properties and the degradation. The environmental degradation of plastics is an important factor for the formation, distribution, and accumulation of microplastics in the aquatic system. Thus, this chapter also summarizes the degradation pathways for synthetic polymers in the environment.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Analysis of Microplastics: Sampling, Sample Preparation, and Identification
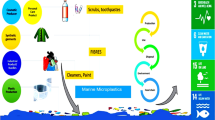
The investigation of small synthetic polymer particles (size<5 mm), so-called microplastics, strongly depends on appropriate analytical methods. These particles are present in the aquatic environment due to mechanical degradation of macroplastics (size>5 mm) or the introduction of man-made microparticles. The analysis of microplastics is a new challenge for analytical scientists. The small size of microplastics complicates their determination in environmental samples compared to macroplastics and demands for more sophisticated analytical approaches. Microplastics are heterogeneously distributed in the environment, and this impedes the representative sampling of sediments and water. The sample matrix, independent of the sampled environmental compartment, contains a high burden of particles of natural origin that strongly interfere with the visual detection of microplastics. Therefore, suitable methods for the sample preparation are needed to extract microplastics and reduce the number of natural particles. Moreover, an analytical method for the identification and confirmation of the plastic particles is mandatory to obtain reliable results. A wide range of different sampling methods, sample treatments, and detection methods were described (Fig. 1).
Possible strategies described in literature for the analysis of microplastics in sediment and water samples starting with the sampling to the report of the results. The sample preparation is split in pretreatment, the density separation, and the posttreatment of the separated microplastics. Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy energy-dispersive X-ray spectroscopy (SEM-EDS), pyrolysis- or thermal desorption-gas chromatography/mass spectrometry (Pyr-GC/MS, TDS-GC/MS) are deployed for the analysis
1.1 Sampling of Microplastics
The sampling of microplastics in the aquatic environment strongly depends on the compartment that is the subject of interest. In general, this can be differentiated between sampling of the aqueous phase (surface water, water column) and the sediment phase (shoreline sediments, riverbed, or lakebed sediments).
1.1.1 Sampling of the Aqueous Phase
The concentrations of microplastics in aqueous samples are relatively low compared to those in the sediments. Therefore, a large volume of the water samples (up to hundreds of liter) is usually filtered during the sampling process to obtain a representative sample. Sampling of the water surface is carried out in most cases with neuston or plankton nets supported by a flow meter to determine the accurate sample volume. These nets are used in different mesh sizes ranging from 50 to 3,000 μm, while 300 μm is the most commonly used mesh size along all studies [1]. This approach leads to nonquantitative sampling of microplastics with particle sizes <300 μm. The nets with smaller mesh sizes are prone to clogging. To overcome this problem, new methods are being developed using filter cascades that result in a size fractionation during the sampling and the reduction of the matrix burden of the small mesh sizes [2].
Less frequently, a sample of the aqueous phase is taken below the water surface. Sampling of the water column is carried out by direct filtration of the water with submersible pumps or is reported by the acquisition of batch samples [3, 4].
1.1.2 Sampling of Sediments
There is no commonly accepted sampling strategy so far for sediment samples. First, the sediments samples must be divided into samples from the shoreline and the river- or lakebed. The collection of bed sediments by sediment grabs provides relatively comparable results due to the standardized sampling instrument [5]. The applications of corers allows the determination of microplastic depth profiles but results in small sample volumes (25 cm3) and is so far only reported for the marine environment [6]. The differences between microplastic studies for the sampling of shore sediments start with the selection of the sampled area. Shore sediments are collected parallel, perpendicular, or randomly selected in different distances to the shoreline. The majority of the studies reports the collection of grid samples with sampling depths of 2–5 cm of the upper sediment layer [7, 8]. Other studies state the sampling in relation to the lowest flotsam line of the waterbody [9, 10]. Sample collection is usually carried out with stainless steel spoons, trowels, or shovels [10, 11]. In addition, the sampling procedure used will affect how the corresponding results are reported. For example, studies that use grid samples usually report the results per surface sampled (e.g., m2), whereas studies based on aerial bulk samples give the results referred to the volume or mass of the collected sample (e.g., m3 or kg).
During the sampling and the sample preparation, it is important to avoid contact with plastic equipment to keep the contamination by the method low. If plastic vessels are included for transportation, blank samples must be also analyzed to quantify their contribution to the microplastic load of the sample [8, 10]. In general, blank samples need to be regarded in microplastic studies to estimate the limit of quantification of each method used, as the limit of quantification (LOQ) is mainly affected by the background contamination [12]. Especially studies dealing with fibers often neglected the analysis of blank samples; thus, the results obtained might be of limited validity. Moreover, the entire method starting from the sample preparation to the analytical detection must be critically evaluated. Therefore, a proper validation must be performed, which also allows a good comparability between different studies. This includes, for example, the determination of within-site variabilities for the sampling process or the determination of recovery rates for the separation methods used during the sample preparation [10, 13, 14].
1.2 Sample Preparation
Even large microplastics like plastic pellets, especially aged and fouled ones, are difficult to distinguish from natural matter in surface water samples with the naked eye. Various methods were developed that allow the mechanical separation of microplastics from the sediment and the removal or reduction of natural debris in the sample prior to analysis of the separated particles. A variety of techniques have been used during the sample treatment and the microplastic identification. Because not all studies conducted extensive method validation including the determination of recovery of the microplastic particles or did not provide experiments with blank samples, the resulting data can lack comparability.
1.2.1 Separation of Microplastics from Sediment Samples
In contrast to microplastics in water, which are easily filtered from the sample during the sampling process, microplastics in sediment samples must be separated in the first step of the sample preparation. A commonly used technique for the separation of plastic particles from sediment particles is the density separation. In a solution of high density, the microplastic particles float, while the very dense sediment particles settle. Numerous different techniques are described in literature, many of them based on the separation introduced by Thompson et al. [15]. Alterations to this method include the use of different salts to create the dense liquid used for separation and the development of different instrumental setups and different pretreatment and posttreatment steps of the samples (compare Fig. 1).
In addition to sodium chloride, which was used by Thompson et al. [15] and others, the application of sodium iodide and zinc chloride has also been reported [16,17,18]. Sodium iodide, sodium tungstate, and zinc chloride offer the possibility to produce solutions with higher densities than sodium chloride. As the density of a saturated sodium chloride solution (ρ ≈ 1.2 g cm−3) is rather limited and does not offer consistent separation of higher density polymers such as polyoxymethylene, polyvinyl chloride (PVC), and polyethylene terephthalate (PET), sodium iodide, sodium polytungstate (ρ ≈ 1.6 g cm−3), and zinc chloride are viable choices. Density separations in the microplastic research rarely use sodium polytungstate despite the possibility of solutions with high density (ρ up to 1.6 g cm−3), as it is too expensive for the application in large volume samples [11]. Sodium iodide (ρ ≈ 1.6–1.8 g cm−3) is usually combined with a pre-separation, based on elutriation that separates less dense particles from heavier particles in an upward directed stream of gas or water. This procedure is necessary to minimize the volume needed for the density separation due to the high costs of sodium iodide [14, 16]. The application of zinc chloride enables solutions with densities of ρ > 1.6–1.7 g cm−3 and is suitable for the separation of most polymer types. Due to the lower costs compared to sodium tungstate and sodium iodide, zinc chloride is frequently reported in recent studies [8]. However, the ecological hazards of zinc chloride complicate the disposal of used solutions and contaminated sediments. Thus, the recycling of solutions containing zinc chloride, sodium iodide, or sodium polytungstate offers a possibility to overcome the waste management problem and reduce the material costs. To improve the effectivity, the repeatability, and the ease of handling for the density separation method, different setups were developed. The initial use of beakers or Erlenmeyer flask was substituted by the use of separation funnels, vacuum-enhanced separation of the plastic particles, or stainless steel separators with high sample volume capacity [9, 10, 13].
Recent developments focus on alternatives to density separation techniques. Elutriation seems to be a suitable and cost-effective alternative even without following density separation, yielding in good recoveries for polymers with densities of up to ρ = 1.4, and the versatility of this method might be improved with a pre-size fractionation of the sample [19].
A different approach includes accelerated solvent extraction (ASE) for the separation of plastics from soils. The extraction by ASE is carried out under higher pressure to increase the boiling point of the extraction solvent, which increases the extraction speed. The process usually uses metal cells of small volume that can resist the pressure. This method bypasses the need for further sample purification and benefits of a high degree of automatization and allows for a quantitative extraction of small plastic particles. However, the identification of extracts consisting of multiple polymer types is complicated, and the size of the extracted sediment sample is limited due to the small size of the extraction cell of the instrument [20].
1.2.2 Removal of Natural Debris
The identification of microplastic particles is often prevented by natural debris that is present in the sample and accompanies the microplastics during the sampling of water samples or the density separation. Thus, the destruction of natural debris or biological material is unavoidable to minimize the possibility of misidentification or underestimation of small plastic particles. The destruction of natural material can be carried out by chemical or enzymatically catalyzed reactions. Chemical destruction of natural debris is achieved through the treatment of the sample with hydrogen peroxide, mixtures of hydrogen peroxide and sulfuric acid, and Fenton-like reactions prior or after the density separation [8, 18, 21]. These harsh conditions might result in losses of plastics that are labile to oxidation or unstable in strong acidic solutions, such as poly(methyl methacrylate) or polycarbonates.
To avoid the loss of synthetic polymers, which are not resistant against acidic treatments, usage of sodium hydroxide was proposed. However, Cole et al. report that the alkaline treatment with sodium hydroxide could damage some of the synthetic polymers as well [22]. Dehaut et al. showed that the application of potassium hydroxide is preferable for the destruction of organic material, as it seems to attack the synthetic polymers less than the abovementioned methods [23].
Enzymatic treatments were developed for biota-rich marine surface water samples, which allow the detection of pH-sensitive polymers [22]. Single-enzyme approaches using proteinase K or mixtures of technical enzymes (lipase amylase, proteinase, chitinase, cellulase) were used for the removal of biological material, as the enzymatic digestion can be carried out under moderate experimental conditions in terms of pH and temperature. Unfortunately, the use of enzymes involves several disadvantages. Enzymatic treatments are, compared to chemical treatments, expensive and very time-consuming and might not result in a complete removal of the natural debris.
1.3 Identification of Microplastics
In most studies, microplastics are first identified visually, before an identification of the polymer type is undertaken. Larger particles can be identified with the naked eye, whereas small microplastics are identified using binocular microscopes or scanning electron microscopy (SEM) [6, 24, 25]. Early studies determined microplastic concentrations after visual inspection of the sample only. Depending on the efficiency of the sample treatment and particle size, the visual identification is considered not state of the art and often insufficient resulting in false-positive results. For this reason, further spectroscopic or spectrometric methods are needed to ensure the unambiguous identification of particles made from synthetic polymers.
Spectroscopic identification methods include Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy. These methods are based on the energy absorption by characteristic functional groups of the polymer particles. For larger particles (approximately >500 μm), FTIR can be carried out using an attenuated transverse reflection (ATR) unit as the particles need to be transferred on the crystal of the ATR unit manually [9, 26]. Coupling of FTIR instruments to microscopes such as reflectance or transmission micro-FTIR allows the detection of smaller microplastics [27]. The use of FTIR microscopy in transmission mode is only applicable for smaller particles or thin films that do not fully absorb the IR beam. Moreover, special filters are required in the sample treatment that are translucent to IR radiation, such as aluminum oxide membranes. Both FTIR-based and Raman-based methods are limited in the minimum particle size that can be determined by the physical diffraction of the light. FTIR measurements in transmittance mode are limited for particles between 10 and 20 μm, while Raman instruments can measure particle with sizes that are one to two orders of magnitude smaller, due to the smaller wavelengths that are applied for the excitation. Identification of the polymers by FTIR and Raman is susceptible to environmentally driven changes of the polymer surface or the additive application during polymer processing. Thus, microbial fouling, soiling, adsorption of humic acids, and colored plastics can interfere with the absorbance, reflection, or excitation of the polymer molecules and might lead to misidentification or totally prevent identification of the particles [28] (for an in-depth discussion on microplastic associated biofilms, see [29]). Besides the identification of the polymer type, visual images of particles enable the determination of particle shape.
The application of pyrolysis-gas chromatography/mass spectrometry (Pyr-GC/MS) allows the simultaneous determination of the polymer type and polymer additives by combustion of the sample and the detection of the thermal degradation products of the polymers [16, 30]. The identification of thermal degradation products serves as a marker that is specific for each polymer. The degradation products are separated by GC prior the detection of their specific mass to charge ratios in the mass spectrometer. In contrast to the spectroscopic techniques, Pyr-GC/MS is a destructive method, preventing any further analysis of the plastic particles. Results obtained through Pyr-GC/MS analysis are usually provided as the mass fraction or mass concentration of plastics. Therefore, the determination of particle counts is not possible due to the combustion of the sample. Thermal desorption GC/MS (TDS-GC/MS) in combination with thermogravimetric analysis (TGA) coupled with a solid-phase adsorber enables higher initial sample sizes compared to Pyr-GC/MS [31]. For this reason, more representative results might be obtained for inhomogeneous samples with complex matrices.
SEM can be coupled with energy-dispersive X-ray spectroscopy (SEM-EDS), which produces high-resolution images of the particles and provides an elemental analysis of the measured objects. For SEM-EDS, the particle surface of the sample is scanned by an electron beam. The contact of the electron beam with the sample surface results in the emission of secondary electrons and element-specific X-ray radiation. Thus, an image of the particle can be created and the elemental composition can be identified by using SEM-EDS. It is, therefore, possible to distinguish between microplastics and particles that are composed of inorganic elements, such as aluminum silicates [32].
Alternatively, hardness tests are reported as inspection of the separated particles. Pressure is applied to the particles by needles or tweezers. This precludes misidentifications of microplastics with fragile carbon or carbonate particles that break during the test and are not removed or formed during the sample treatment [33]. However, these tests are very time-consuming, do not provide exact polymer identification, and are less accurate as other instrumental methods.
More specialized but promising approaches for the detection of microplastics are described by Sgier et al. and Jungnickel et al. [34, 35]. The latter describe the measurement and identification of microplastics by time-of-flight secondary ion mass spectrometry. An imaging technique allows the visualization of the particles, and the ionization of the polymer molecules is carried out by a primary ion source, generating secondary ions of polymer fragments. As with Raman microscopy, this technique enables the identification of particles smaller than 10 μm. Sgier et al. detected microplastics using flow cytometry combined with visual stochastic network embedding (viSNE). viSNE is a tool for the visualization of high-dimensional cytometry data by nonlinear dimension reduction onto two dimensions [36]. This method was capable of detecting microplastic particles directly in environmental samples by using nonbiological reference data sets for the interpretation of the viSNE analysis, although the reliability of the microplastic identification needs to be proven in future studies.
2 Occurrence in the Aquatic Environment
Microplastic particles are present in surface water, sediments, and oceans all over the world, for example, at the Italian, Singapore, and Portuguese coast, at beaches of Hawaii, and islands of the equatorial Western Atlantic as well as at shores of German and Greek islands [3, 11, 17, 37,38,39,40]. First reports of smaller plastic items were primarily focused on plastic pellets that are used in the production of bulk plastic items. Plastic pellets have been quantified on numerous beaches and coastlines, for instance, in New Zealand, Lebanon, and Spain [41,42,43]. However, industrial plastic pellets only compose a small fraction of the numerous microscopic plastic fragments present in the ocean and other aquatic systems [15]. Monitoring studies often subdivide microplastics into categories of spheres, fibers, foams, and fragments and report range in concentrations by up to four orders of magnitude, spanning 1.3 particles kg−1 (German island) over 13.5 particles kg−1 (equatorial Western Atlantic) to 2175 particles kg−1 (Italy). All these studies were carried out in the marine environment, and freshwater systems have attracted less attention until 2010.
In freshwater environments of lakes and rivers, studies also report highly heterogeneous concentrations comparable to those reported for the marine environment. High within-site variabilities, as well as different units used for microplastic quantitation, complicate the comparison of microplastic concentrations in aquatic systems. Microplastics in riverine systems were reported for large European rivers, e.g., the river Rhine and the river Danube, as well as for tributaries such as the river Main. Plastic particles in the river Danube were determined with high abundance and even exceeded the number of fish larvae. Lechner et al. stated that the river might transport high loads of plastic particles into the Black Sea [44]. Studies investigating the river Rhine showed high abundances of microplastics, especially in the German section of the river. Average concentrations amounted to approx. 900,000 particles km−2 for surface water and up to 4000 particles kg−1 for shore sediments. In the Swiss part of the rivers Rhône, Aubonne, Venoge, and Vuachière, microplastics were detected in concentrations between 0.10 and 64 particles m3 (mean, 7 particles m−3; median, 0.36 particles m−3) [45]. Distinctly higher concentrations were detected in Chinese river estuaries. The estuaries of the rivers Jiaojiang, Oujiang, and Minjiang, which all are located in an urban region, contained microplastics in the range of 100–4100 particles m−3 [46].
A study conducted with lakeshore sediments collected from Lake Garda (Italy) showed high abundances of polyethylene (PE) and polystyrene (PS) microplastics, indicating the importance of buoyant microplastics for shore sediments. In addition, polymer particles of a higher density, such as PVC and PET, were also identified in this study underlining the variety of microplastics present in shore sediments [8]. Microplastics were detected in concentrations between 108 and 1,108 particles m−2 with notable spatial variation between the south and the north shore of the lake. Faure et al. reported microplastics in the Swiss parts of Lake Geneva, Lake Constance, Lake Neuchâtel, Lake Maggiore, Lake Zurich, and Lake Brienz. The concentrations of microplastics in lakeshore sediments varied between 20 and 7,200 particles m−2 (mean, 1,300 particles m−2; median, 270 particles m−2) and are comparable with concentrations reported for Lake Garda. Between 11,000 and 220,000 particles km−2 of microplastics were detected in the surface water of the six lakes mentioned. Microplastics were also detected in the Laurentian Great Lakes in North America with concentrations ranging between 0 and 466,305 particles m−2 (mean, 42,533 particles m−2; median, 5,704 particles m−2). Higher abundances of microplastics have been detected in proximity to urban areas [32]. The determination of microplastics in gastrointestinal tract of fish from Lake Victoria (Tanzania) shows first evidence for the microplastic pollution of African lakes [47] (microplastic uptake biological interactions are discussed in Scherer et al. [48] of this volume). A monitoring study of Lake Taihu in China detected up to 6,000,000 particles km−2 in surface water samples. In bulk water samples, microplastics were present in concentrations between 3 and 26 particles L−1, while the sediments of Lake Taihu contained microplastics in the range of 11–235 particles kg−1. The reported concentrations of microplastics in the sediments of Lake Taihu are lower compared to those detected in the sediments of the European lakes. However, it needs to be taken into account that lake-bottom sediments were sampled in Lake Taihu, whereas shore sediments were studied in the European lakes [49]. Lower concentrations of plastic particles in Asian freshwaters were detected in the surface water samples of Lake Hovsgol (Mongolia). Microplastics were quantified in the range of 997–4435 particles km−2, but it should be taken into account that the catchment area of Lake Hovsgol is less populated compared to the abovementioned lakes [50]. For case study discussions on microplastic occurrence in African and Asian freshwaters, see Kahn et al. [51] and Chenxi et al. [52] of this volume.
3 Environmental Degradation of Synthetic Polymers
One of the reasons for the great versatility of many synthetic polymers is their high resistance against environmental influences. However, this fact leads to extremely low degradation and long residence times for synthetic polymers once they enter the environment. Degradation of synthetic polymers can generally be classified as biotic or abiotic, following different mechanisms, depending on a variety of physical, chemical, or biological factors. During the degradation process, polymers are converted into smaller molecular units (e.g., oligomers, monomers, or chemically modified versions) and possibly are completely mineralized [53]. The most important processes for the degradation of synthetic polymers can be divided into (Fig. 2):
-
Physical degradation (abrasive forces, heating/cooling, freezing/thawing, wetting/drying)
-
Photodegradation (usually by UV light)
-
Chemical degradation (oxidation or hydrolysis)
-
Biodegradation by organisms (bacteria, fungi, algae)
Mechanical degradation is an important factor with regard to plastics in the aquatic environment. In most cases, aging of the polymer by environmental influences, such as photodegradation or chemical degradation of additives, changes the polymer properties and leads to embrittlement of the polymer [54]. The recalcitrant material is then shredded into smaller particles by friction forces occurring during the movement through different environmental habitats (also see Kooi et al. [55] of this volume for a discussion on microplastics fate and transportation). This degradation generally leads to smaller plastic particles, which can result in particles with sizes between 1 and 5,000 μm. Such particles are classified as microplastics. However, the mechanical degradation does not stop if the particles are within the size range of microplastics. Thus, the formation of even smaller particles, so-called nanoplastics, is very likely [56]. These nanoplastics could have different properties compared to the original macroplastics or microplastics (for a discussion on nanomaterials, see Rist and Hartmann [57] of this volume). In both cases, the mechanical degradation leads to a decrease in particle size and consequently to an increase in the surface area of the polymer particles, which results in faster degradation due to higher reactivity.
Under normal environmental conditions in aquatic systems, the temperature is not high enough to start chemical changes of synthetic polymers; thus, thermal degradation is not significant for freshwaters [58, 59].
The degradation of synthetic polymers in the environment on a molecular basis is usually initiated by photooxidation (with UV radiation) or by hydrolysis and is eventually followed by chemical oxidation [60]. The predominant mechanisms strongly depend on the type of polymer, as there are numerous different compositions of synthetic polymers produced (i.e., polyolefins, polyesters, polyamides). After the initial reactions, the molecular weight of the polymer is decreased, and the reacted groups become available for microbial degradation. Photooxidation is usually a fast process, but the degradation rate also depends on the extent of additives in a particular polymer that could prevent oxidation processes (i.e., antioxidants). Moreover, the photodegradation of plastics floating in the aquatic environment is slower compared to degradation in terrestrial exposure [61]. Experiments on the disintegration of PE and PS showed faster degradation on the water surface compared to plastics that partially or completely submerged, likely related to the decreasing intensity of light and thus to the lower rate of photooxidation [62]. For this reason, many plastics can stay in the aquatic environment for decades or hundreds of years.
Biodegradation of synthetic polymers can occur in two different environments (aerobic and anaerobic). The extent of the degradation of polymers into CO2, H2O, N2, H2, CH4, salts, minerals, and biomass (mineralization) can be full or partial [63]. Partial or primary degradation of the polymer chain leads to stable or temporarily stable transformation products. Biodegradation is coupled to three essential criteria:
-
1.
Microorganisms must be present that can depolymerize the target substance and mineralize the monomeric compounds with enzymes of an appropriate metabolic pathway.
-
2.
The environmental parameters, such as temperature, pH, moisture, and salinity must provide conditions that are necessary for biodegradation.
-
3.
The morphology of polymer particles must render the attachment of microorganisms and the formation of a biofilm, while the structure of the polymeric substrate, e.g., chemical bonds, degree of polymerization, degree of branching, and parameter, such as hydrophobicity or crystallinity, must not hinder microbial actions.
Since the size of synthetic polymers is generally too large to penetrate the cell membranes of microorganisms, the first step of biotic degradation is the cleavage of side chains or the polymer backbone and the formation of smaller polymer units (monomers, oligomers) by extracellular enzymes [64]. In most cases, this first step of depolymerization involves an enzymatically catalyzed hydrolysis of amides, esters, or urethane bonds. These smaller molecules can then be absorbed by microorganisms and metabolized. Of course, abiotic hydrolysis can also result in intermediates that are then further metabolized by microorganisms [65].
The complete biotic degradations of poly(ε-caprolactam) and water-soluble polyethylene glycol are well described in literature [66]. However, most of the plastics occurring in the environment are water insoluble, and many of the synthetic polymers present in the aquatic environment, such as PE, polypropylene (PP), PS, and PET, degrade very slowly or not at all. The degradation of these polymers is usually a combination of abiotic and biotic degradation pathways.
Polyolefins, such as PE and PP, represent a class of substances with high industrial production volumes and are determined frequently in environmental samples. These polymers are usually not biodegradable, as the alkyl backbone is not accessible for microorganism and must undergo an abiotic transformation. The alkyl backbone of polyolefins offers a high resistivity against hydrolysis but is usually susceptible to oxidative degradation. To prevent this, additives are added during the production process, and the oxidative or photooxidative degradation of the polymer is delayed until the antioxidants are consumed. After the initial oxidation of the surface of polyolefins, the degradation could occur in several weeks but results in the formation of microplastics as possible intermediates [67]. These smaller and oxidized plastic fragments are more susceptible to microbial attack, e.g., biodegradation of PE is described for pre-oxidized fragments of the original material by Pseudomonas sp. [68].
4 Conclusion
The results of studies worldwide highlight the great importance of microplastics for freshwater ecosystems, as they are present in high abundance. Microplastics are emerging contaminants in the aquatic environment, and attention should be focused on a harmonized nomenclature of microplastic particles with official guidelines for microplastic studies. The definition of microplastics often remains vague, and different size classes are investigated in monitoring studies. For a thorough investigation of microplastic pollution, standardized methods, especially for the sampling of the different compartments, are a key factor for meaningful results. The quantitation of microplastics differs due to the individual sampling method and studies still lacking in comparability. Therefore, future studies should integrate additional units to describe microplastic occurrence in their investigations. Moreover, an integral step of future investigations should be a sufficient validation of the microplastic analysis, as different technological approaches might be used or future technological improvements will be implemented for the determination of microplastics. Important approaches, such as Raman-, FTIR-, and GC/MS-based techniques, seem to be very versatile for the detection of microplastics, but a better comparability of results obtained with either of these methods needs to be established. Degradation of larger plastics will result in a constant source for new microplastic particles of all size classes, and thus, as it is a slow process, microplastics will be a relevant long-term threat.
References
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46(6):3060–3075. doi:10.1021/es2031505
Löder MGJ, Gerdts G (2015) Methodology used for the detection and identification of microplastics—a critical appraisal. In: Bergmann M, Gutow L, Klages M (eds) Marine anthropogenic litter. Springer International Publishing, Cham, pp 201–227. doi:10.1007/978-3-319-16510-3_8
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Mar Pollut Bull 52(7):761–767. doi:10.1016/j.marpolbul.2005.11.017
Norén F, Naustvoll L-J (2010) Survey of microscopic anthropogenic particles in Skagerrak. Commissioned by KLIMA – Og Forurensningsdirektoratet
Castañeda RA, Avlijas S, Simard MA, Ricciardi A (2014) Microplastic pollution in St. Lawrence River sediments. Can J Fish Aquat Sci 71(12):1767–1771. doi:10.1139/cjfas-2014-0281
Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environ Pollut 182:495–499. doi:10.1016/j.envpol.2013.08.013
Zbyszewski M, Corcoran PL, Hockin A (2014) Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J Great Lakes Res 40(2):288–299. doi:10.1016/j.jglr.2014.02.012
Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23(19):R867–R868. doi:10.1016/j.cub.2013.09.001
Browne MA, Galloway TS, Thompson RC (2010) Spatial patterns of plastic debris along Estuarine shorelines. Environ Sci Technol 44(9):3404–3409. doi:10.1021/es903784e
Klein S, Worch E, Knepper TP (2015) Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ Sci Technol 49(10):6070–6076. doi:10.1021/acs.est.5b00492
Corcoran PL, Biesinger MC, Grifi M (2009) Plastics and beaches: a degrading relationship. Mar Pollut Bull 58(1):80–84. doi:10.1016/j.marpolbul.2008.08.022
Leslie H, Van Velzen M, Vethaak A (2013) Microplastic survey of the Dutch environment. Novel data set of microplastics in North Sea sediments, treated wastewater effluents and marine biota Amsterdam. Institute for Environmental Studies, VU University, Amsterdam
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 10:524–537. doi:10.4319/lom.2012.10.524
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Mar Pollut Bull 70(1–2):227–233. doi:10.1016/j.marpolbul.2013.03.009
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304(5672):838–838. doi:10.1126/science.1094559
Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. doi:10.1016/j.envpol.2013.07.027
Vianello A, Boldrin A, Guerriero P, Moschino V, Rella R, Sturaro A, Da Ros L (2013) Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar Coast Shelf Sci 130:54–61. doi:10.1016/j.ecss.2013.03.022
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89(1):213–217. doi:10.1007/s00128-012-0642-7
Kedzierski M, Le Tilly V, Bourseau P, Bellegou H, Cesar G, Sire O, Bruzaud S (2016) Microplastics elutriation from sandy sediments: a granulometric approach. Mar Pollut Bull 107(1):315–323. doi:10.1016/j.marpolbul.2016.03.041
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50(11):5774–5780. doi:10.1021/acs.est.6b00816
Yonkos LT, Friedel EA, Perez-Reyes AC, Ghosal S, Arthur CD (2014) Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ Sci Technol 48(24):14195–14202. doi:10.1021/es5036317
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep 4:4528. doi:10.1038/srep04528
Dehaut A, Cassone A-L, Frère L, Hermabessiere L, Himber C, Rinnert E, Rivière G, Lambert C, Soudant P, Huvet A, Duflos G, Paul-Pont I (2016) Microplastics in seafood: benchmark protocol for their extraction and characterization. Environ Pollut 215:223–233. doi:10.1016/j.envpol.2016.05.018
Karapanagioti HK, Klontza I (2007) Investigating the properties of plastic resin pellets found in the coastal areas of lesvos island. Global Nest J 9(1):71–76
Gilfillan LR, Ohman MD, Doyle MJ, Watson W (2009) Occurrence of plastic micro-debris in the Southern California current system. Cal Coop Ocean Fish 50:123–133
Doyle MJ, Watson W, Bowlin NM, Sheavly SB (2011) Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar Environ Res 71(1):41–52. doi:10.1016/j.marenvres.2010.10.001
Harrison JP, Ojeda JJ, Romero-Gonzalez ME (2012) The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci Total Environ 416:455–463. doi:10.1016/j.scitotenv.2011.11.078
Rocha-Santos T, Duarte AC (2015) A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal Chem 65:47–53. doi:10.1016/j.trac.2014.10.011
Harrison JP, Hoellein TJ, Sapp M, Tagg AS, Ju-Nam Y, Ojeda JJ (2017) Microplastic-associated biofilms: a comparison of freshwater and marine environments. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_9 (in this volume)
Trimpin S, Wijerathne K, McEwen CN (2009) Rapid methods of polymer and polymer additives identification: multi-sample solvent-free MALDI, pyrolysis at atmospheric pressure, and atmospheric solids analysis probe mass spectrometry. Anal Chim Acta 654(1):20–25. doi:10.1016/j.aca.2009.06.050
Dümichen E, Barthel A-K, Braun U, Bannick CG, Brand K, Jekel M, Senz R (2015) Analysis of polyethylene microplastics in environmental samples, using a thermal decomposition method. Water Res 85:451–457. doi:10.1016/j.watres.2015.09.002
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77(1–2):177–182. doi:10.1016/j.marpolbul.2013.10.007
Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9(12):e111913. doi:10.1371/journal.pone.0111913
Sgier L, Freimann R, Zupanic A, Kroll A (2016) Flow cytometry combined with viSNE for the analysis of microbial biofilms and detection of microplastics. Nat Commun 7:11587. doi:10.1038/Ncomms11587
Jungnickel H, Pund R, Tentschert J, Reichardt P, Laux P, Harbach H, Luch A (2016) Time-of-flight secondary ion mass spectrometry (ToF-SIMS)-based analysis and imaging of polyethylene microplastics formation during sea surf simulation. Sci Total Environ 563:261–266. doi:10.1016/j.scitotenv.2016.04.025
Amir E-AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D (2013) viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol 31(6):545–552. doi:10.1038/nbt.2594
Martins J, Sobral P (2011) Plastic marine debris on the Portuguese coastline: a matter of size? Mar Pollut Bull 62(12):2649–2653. doi:10.1016/j.marpolbul.2011.09.028
Ivar do Sul JA, Spengler Â, Costa MF (2009) Here, there and everywhere. Small plastic fragments and pellets on beaches of Fernando de Noronha (Equatorial Western Atlantic). Mar Pollut Bull 58(8):1236–1238. doi:10.1016/j.marpolbul.2009.05.004
Dekiff JH, Remy D, Klasmeier J, Fries E (2014) Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ Pollut 186:248–256. doi:10.1016/j.envpol.2013.11.019
Karapanagioti HK, Endo S, Ogata Y, Takada H (2011) Diffuse pollution by persistent organic pollutants as measured in plastic pellets sampled from various beaches in Greece. Mar Pollut Bull 62(2):312–317. doi:10.1016/j.marpolbul.2010.10.009
Gregory MR (1978) Accumulation and distribution of virgin plastic granules on New Zealand beaches. N Z J Mar Freshw Res 12(4):399–414. doi:10.1080/00288330.1978.9515768
Shiber JG (1979) Plastic pellets on the coast of Lebanon. Mar Pollut Bull 10(1):28–30. doi:10.1016/0025-326X(79)90321-7
Shiber JG (1987) Plastic pellets and tar on Spain’s Mediterranean beaches. Mar Pollut Bull 18(2):84–86. doi:10.1016/0025-326X(87)90573-X
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181. doi:10.1016/j.envpol.2014.02.006
Faure F, Demars C, Wieser O, Kunz M, de Alencastro LF (2015) Plastic pollution in Swiss surface waters: nature and concentrations, interaction with pollutants. Environ Chem 12(5):582–591. doi:10.1071/EN14218
Zhao SY, Zhu LX, Li DJ (2015) Microplastic in three urban estuaries, China. Environ Pollut 206:597–604. doi:10.1016/j.envpol.2015.08.027
Biginagwa FJ, Mayoma BS, Shashoua Y, Syberg K, Khan FR (2016) First evidence of microplastics in the African Great Lakes: recovery from Lake Victoria Nile perch and Nile tilapia. J Great Lakes Res 42(1):146–149. doi:10.1016/j.jglr.2015.10.012
Scherer C, Weber A, Lambert S, Wagner M (2017) Interactions of microplastics with freshwater biota. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer Nature, Heidelberg. doi:10.1007/978-3-319-61615-5_8 (in this volume)
Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H (2016) Microplastics in Taihu Lake, China. Environ Pollut 216:711–719. doi:10.1016/j.envpol.2016.06.036
Free CM, Jensen OP, Mason SA, Eriksen M, Williamson NJ, Boldgiv B (2014) High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull 85(1):156–163. doi:10.1016/j.marpolbul.2014.06.001
Khan FR, Mayoma BS, Biginagwa FJ, Syberg K (2017) Microplastics in inland African waters: presence, sources and fate. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_6 (in this volume)
Chenxi W, Kai Z, Xiong X (2017) Microplastic pollution in inland waters focusing on Asia. In: Wagner M, Lambert S (eds) Freshwater Microplastics: Emerging Environmental Contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_5 (in this volume)
Eubeler JP, Zok S, Bernhard M, Knepper TP (2009) Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trends Anal Chem 28(9):1057–1072. doi:10.1016/j.trac.2009.06.007
Duwez AS, Nysten B (2001) Mapping aging effects on polymer surfaces: specific detection of additives by chemical force microscopy. Langmuir 17(26):8287–8292. doi:10.1021/la0113623
Kooi M, Besseling E, Kroeze C, van Wezel AP, Koelmans AA (2017) Modeling the fate and transport of plastic debris in freshwaters: review and guidance. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_7 (in this volume)
Lambert S, Wagner M (2016) Formation of microscopic particles during the degradation of different polymers. Chemosphere 161:510–517. doi:10.1016/j.chemosphere.2016.07.042
Rist SE, Hartmann NB (2017) Aquatic ecotoxicity of microplastics and nanoplastics - lessons learned from engineered nanomaterials. In: Wagner M, Lambert S (eds) Freshwater microplastics: emerging environmental contaminants? Springer, Heidelberg. doi:10.1007/978-3-319-61615-5_2 (in this volume)
Anderson DA, Freeman ES (1961) The kinetics of the thermal degradation of polystyrene and polyethylene. J Polym Sci 54(159):253–260. doi:10.1002/pol.1961.1205415920
McNeill IC, Leiper HA (1985) Degradation studies of some polyesters and polycarbonates—2. Polylactide: degradation under isothermal conditions, thermal degradation mechanism and photolysis of the polymer. Polym Degrad Stab 11(4):309–326. doi:10.1016/0141-3910(85)90035-7
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Andrady AL, Pegram JE, Song Y (1993) Studies on enhanced degradable plastics. II. Weathering of enhanced photodegradable polyethylenes under marine and freshwater floating exposure. J Environ Polym Degrad 1(2):117–126. doi:10.1007/BF01418205
Leonas KK, Gorden RW (1993) An accelerated laboratory study evaluating the disintegration rates of plastic films in simulated aquatic environments. J Environ Polym Degrad 1(1):45–51. doi:10.1007/bf01457652
Grima S, Bellon-Maurel V, Feuilloley P, Silvestre F (2000) Aerobic biodegradation of polymers in solid-state conditions: a review of environmental and physicochemical parameter settings in laboratory simulations. J Polym Environ 8(4):183–195. Unsp 1566-2543/00/1000-0183/0. doi:10.1023/A:1015297727244
Gu J-G, Gu J-D (2005) Methods currently used in testing microbiological degradation and deterioration of a wide range of polymeric materials with various degree of degradability: a review. J Polym Environ 13(1):65–74. doi:10.1007/s10924-004-1230-7
Müller R-J, Kleeberg I, Deckwer W-D (2001) Biodegradation of polyesters containing aromatic constituents. J Biotechnol 86(2):87–95. doi:10.1016/S0168-1656(00)00407-7
Eubeler JP, Bernhard M, Knepper TP (2010) Environmental biodegradation of synthetic polymers II. Biodegradation of different polymer groups. Trac, Trends Anal Chem 29(1):84–100. doi:10.1016/j.trac.2009.09.005
Weinstein JE, Crocker BK, Gray AD (2016) From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ Toxicol Chem 35(7):1632–1640. doi:10.1002/etc.3432
Reddy MM, Deighton M, Gupta RK, Bhattacharya SN, Parthasarathy R (2009) Biodegradation of oxo-biodegradable polyethylene. J Appl Polym Sci 111(3):1426–1432. doi:10.1002/app.29073
Acknowledgments
We would like to thank the German Federal Ministry for Education and Research (BMBF) in the frame of the project microplastics in the water cycle (MiWa; FKZ: 02WRS1378D).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Klein, S., Dimzon, I.K., Eubeler, J., Knepper, T.P. (2018). Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Environment. In: Wagner, M., Lambert, S. (eds) Freshwater Microplastics . The Handbook of Environmental Chemistry, vol 58. Springer, Cham. https://doi.org/10.1007/978-3-319-61615-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-61615-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61614-8
Online ISBN: 978-3-319-61615-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)