Abstract
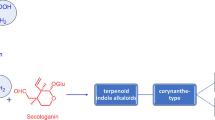
Terpenoids are the most functionally and structurally varied group of plant metabolites. These are synthesized in all organisms but are especially abundant and diverse in plants. Despite their diversity of functions and structures, all terpenoids are derived from the common five-carbon (C5) building unit, isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) through MEP pathway in all living organisms. The MEP pathway synthesizes IPP and DMAPP in plastids in plants and more complex terpenoids are usually formed by “head-to-tail” or “head-to-head” addition of isoprene units. Monoterpene indole alkaloids (MIAs) are one of important class of terpenoids which are C10 terpenoids, consisting of two isoprene units and are the components of more than 2000 defined compounds. These are a large group of plant-derived natural products with a range of pharmacological properties. These MIAs are potent drugs, such as anticancer, antimalarial, and antiarrhythmic agents. These MIAs have been found in eight different plant families, being most common in Apocynaceae, Rubiaceae, and Loganiaceae. Madagascar periwinkle, Catharanthus roseus, the best-characterized MIA-producing plant species, is the source of the valuable MIAs. It has approximately 130 alkaloids of indole group, out of which 25 are dimeric in nature. Some of these compounds have important medicinal properties such as anticancerous property (vincristine and vinblastine) and antiarrhythmic property (ajmalicine and serpentine). In the present chapter, molecular network of monoterpene indole alkaloids (MIAs) signaling in plants with reference to Catharanthus roseus (L.) G. Don has been reviewed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Aerts RJ, Gisi D, De Carolis E, De Luca V, Baumann TW (1994) Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus roseus and Cinchona seedlings. Plant J 5:635–643
Aerts RJ, Schafer A, Hesse M, Baumann TW, Slusarenko A (1996) Signalling molecules and the synthesis of alkaloids in Catharanthus roseus seedlings. Phytochemistry 42:417–422
Araki N, Kusumi K, Masamoto K, Niwa Y, Iba K (2000) Temperature-sensitive Arabidopsis mutant defective in 1-deoxy-D-xylulose 5-phosphate synthase within the plastid non-mevalonate pathway of isoprenoid biosynthesis. Physiol Plant 108:19–24
Arvy MP, Imbault N, Naudascher F, Thiersault M, Doireau P (1994) 2,4-D and alkaloid accumulation in periwinkle cell suspensions. Biochimie 76:410–416
Bach TJ (1995) Some aspects of isoprenoid biosynthesis in plants- A review. Lipids 30:191–202
Back K, Chappell J (1996) Identifying functional domains within terpene cyclases using a domain-swapping strategy. Proc Natl Acad Sci U S A 93(13):6841–6845
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascade. Plant Cell 12:1103–1115
Besseau S, Kellner F, Lanoue A, Thamm AMK, Salim V, Schneider B, Geu-Flores F, Hofer R, Guirimand G, Guihur A, Oudin A, Glevarec G, Foureau E, Papon N, Clastre M, Giglioli-Guivarch N, St-Pierre B, Werck-Reichhart D, Burlat V, De Luca V, O’Connor SE, Courdavault V (2013) A Pair of Tabersonine 16-Hydroxylases initiates the synthesis of vindoline in an organ-dependent manner in Catharanthus roseus. Plant Physiol 163(4): 1792–1803
Binder BYK, Christie AM, Jacqueline P, Shanks V, San KY (2009) The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol Prog 25:861–865
Blom TJM, Sierra M, van Vliet TB, Franke-van Dijk MEI, de Koning P, van Iren F, Verpoorte R, Libbenga KR (1991) Uptake and accumulation of ajmalicine into isolated vacuoles of cultured cells of Catharanthus roseus (L.) G. Don and its conversion into serpentine. Planta 183:170–177
Bohlmann J, Lins T, Martin W, Eilert U (1996) Anthranilate synthase from Ruta graveolens. Duplicated ASα genes encode tryptophan-sensitive and tryptophan-insensitive isoenzymes specific to amino acid and alkaloid biosynthesis. Plant Physiol 111:507–514
Bongaerts J, Kramer M, Muller U, Raeven L, Wubbolts M (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3:289–300
Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40:188–199
Bouvier F, Suire C, d’Harlingue A, Backhaus RA, Camara B (2000) Molecular cloning of geranyl diphosphate synthase and compartmentation of monoterpene synthesis in plant cells. Plant J 24:241–252
Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B (2004) Co-expression of the three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid derived primary metabolites. Plant J 38:131–141
Caelles C, Ferrer A, Balcells L, Hegardt FG, Boronat A (1989) Isolation and structural characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Mol Biol 13:627–638
Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepción M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-d-erythritol 4-phosphate pathway. Plant Physiol 129:1581–1590
Chahed K, Oudin A, Guivarch N, Hamdi S, Chenieux JC, Rideau M, Clastre M (2000) 1-Deoxy-D-xylulose 5-phosphate synthase from periwinkle: cDNA identification and induced gene expression in terpenoid indole alkaloid-producing cells. Plant Physiol Biochem 38:559–566
Choi YH, Tapias EC, Kim HK, Alfons WML, Erkelens C, Jacobus TJ, Berzin J, Zel J, Verpoorte R (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135:2398–2410
Collu G, Unver N, Peltenburg-Looman AMG, Van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220
Collu G, Garcia AA, van der Heijden R, Verpoorte R (2002) Activity of the cytochrome P450 enzyme geraniol 10-hydroxylase and alkaloid production in plant cell cultures. Plant Sci 162:165–172
Contin A, Van der Heijden R, Lefeber AWM, Verpoorte R (1998) The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett 434:413–416
Contin A, Collu G, van der Heijden R, Verpoorte R (1999) The effects of phenobarbital and ketonazole on the alkaloid biosynthesis in Catharanthus roseus cell suspension cultures. Plant Physiol Biochem 37:139–144
Cordier H, Karst F, Berges T (1999) Heterologous expression in Saccharomyces cerevisiae of an Arabidopsis thaliana cDNA encoding mevalonate diphosphate decarboxylase. Plant Mol Biol 39:953–967
Cordoba E, Salmi M, Leon P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 60:2933–2943
Costa MMR, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, Memelink J, Barcelo AR, Sottomayor M (2008) Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol 146(2):403–417
Cunningham FX, Lafond TP, Gantt E (2000) Evidence of a role for LytB in the non mevalonate pathway of isoprenoid biosynthesis. J Bacteriol 182:5841–5848
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109(1):69–75, PMCID: PMC1240543
Davies WJ, Jones HG (1991) Abscisic acid physiology and biochemistry. Bioscientific Publishers, Oxford
De Carolis E, Chan F, Balsevich J, De Luca V (1990) Isolation and characterization of a 2-oxoglutarate dependent dioxygenase involved in the second-to-last step in vindoline biosynthesis. Plant Physiol 94:1323–1329
De Carolis E, De Luca V (1993) Purification, characterization, and kinetic analysis of a 2-oxoglutarate-dependent dioxygenase involved in vindoline biosynthesis from Catharanthus roseus. J Biol Chem 268:5504–5511
De Luca V, Laflamme P (2001) The expanding universe of alkaloid biosynthesis. Curr Opin Plant Biol 4:225–233
De Luca V, Balsevich J, Tyler RT, Eilert U, Panchuk BD, Kurz WGW (1986) Biosynthesis of indole alkaloids: developmental regulation of the biosynthetic pathway from tabersonine to vindoline in Catharanthus roseus. J Plant Physiol 125:147–156
De Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci U S A 86(8):2582–2586
De Waal A, Meijer AH, Verpoorte R (1995) Strictosidine synthase from Catharanthus roseus: purification and characterization of multiple forms. Biochem J 306(2):571–580
Decendit A, Liu D, Ouelhazi L, Doireau P, Merillon JM, Rideau M (1992) Cytokinin-enhanced accumulation of indole alkaloids in Catharanthus roseus cell cultures—the factors affecting the cytokinin response. Plant Cell Rep 11:400–403
Decendit A, Petit G, Andreu F, Doireau P, Merillon JM, Rideau M (1993) Putative sites of cytokinin action during their enhancing effect on indole alkaloid accumulation in periwinkle cell suspensions. Plant Cell Rep 12:710–712
Dethier M, De Luca V (1993) Partial purification of an N-methyltransferase involved in vindoline biosynthesis in Catharanthus roseus. Phytochemistry 32: 673–678
Dewick PM (2002) Medicinal natural products: a biosynthetic approach, 2nd edn. Wiley, Chichester, pp 291–403
Dumontet C, Sikic BI (1999) Mechanisms of action of and resistance to anti tubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol 17:1061–1070
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tissue Organ Cult 68:265–270
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
El-Sayed M, Choi YH, Frederich M, Roytrakul S, Verpoorte R (2004) Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine. Biotechnol Lett 26:793–798
Enfissi EMA, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J 3:17–27
Facchini PJ (2001) Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol 52:29–66
Facchini PJ, DiCosmo F (1991) Secondary metabolites biosynthesis in cultured cells of Catharanthus roseus (L.) Don immobilized by adhesion to glass fibers. Appl Microbiol Biotechnol 35:382–392
Fahn W, Gundlach H, Deus-Neumann B, Stockigt J (1985) Late enzymes of vindoline biosynthesis: acetyl-CoA:17-O-deacetylvindoline 17-O-acetyl- transferase. Plant Cell Rep 4:337–340
Gantet P, Memelink J (2002) Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci 23:563–569
Geerlings A, Martinez-Lozano IM, Memelink J, van der Heijden R, Verpoorte R (2000) Molecular cloning and analysis of strictosidine β-D-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem 275:3051–3056
Goddijn OJ, Lohman FP, de Kam RJ, Schilperoort RA, Hoge JH (1994) Nucleotide sequence of the tryptophan decarboxylase gene of Catharanthus roseus and expression of tdc-gusA gene fusions in Nicotiana tabacum. Mol Gen Genet 242(2):217–225
Goodbody AE, Endo T, Vukovic J, Kutney JP, Choi LSL, Misawa M (1988) Enzymatic coupling of catharanthine and vindoline to form 3,4-anhydrovinblastine by horseradish peroxidase. Planta Med 54:136–140
Guirimand G, Burlat V, Oudin A, Lanoue A, St-Pierre B, Courdavault V (2009) Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the subcellular localization of hydroxyl methylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Rep 28:1215–1234
Guirimand G, Guihur A, Ginis O, Poutrain P, Héricourt F, Oudin A, Lanoue A, St-Pierre B, Burlat V, Courdavault V (2011) The subcellular organization of strictosidine biosynthesis in Catharanthus roseus epidermis highlights several trans-tonoplast translocations of intermediate metabolites. FEBS Lett 278:749–763
Guirimand G, Guihur A, Phillips MA, Oudin A, Glévarec G, Mahroug S, Melin C, Papon N, Clastre M, Giglioli-Guivarc’h N, St-Pierre B, Rodríguez- Concepción M, Burlat V, Courdavault V (2012) Triple subcellular targeting of isopentenyl diphosphate isomerases encoded by a single gene. Plant Signal Behav 7(11):1495–1497
Gupta P, Goel R, Pathak S, Srivastava A, Singh SP, Sangwan RS, Asif MH, Trivedi PK (2013) De Novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS One 8(5):e62714, PMCID: PMC3648579
Hallahan DL, West JM, Wallsgrove RM, Smiley DW, Dawson GW, Pickett JA, Hamilton JG (1995) Purification and characterization of an acyclic monoterpene primary alcohol: NADP+ oxidoreductase from catmint (Nepeta racemosa). Arch Biochem Biophys 318:105–112
Hemscheidt T, Zenk MH (1985) Partial purification and characterization of a NADPH dependent tetrahydroalstonine synthase from Catharanthus roseus cell suspension cultures. Plant Cell Rep 4:216–219
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Hilliou F, Costa M, Almeida I, Lopes Cardoso I, Leech M, Ros Barcelo A, Sottomayor M (2002) Cloning of a peroxidase enzyme involved in the biosynthesis of pharmaceutically active terpenoid indole alkaloids in Catharanthus roseus (L.) G. Don. In Acosta M, Rodriguez-Lopez JN, Pedreno MA (eds) Proceedings of the VI international plant peroxidase symposium, University of Murcia and University of A Coruna, pp 152–158
Hong SB, Hughes EH, Shanks JV, San K-Y, Gibson SI (2003) Role of the non-mevalonate pathway in indole alkaloid production by Catharanthus roseus hairy roots. Biotechnol Prog 19(3):1105–1108 (PMID: 12790690)
Hsiao YY, Jeng MF, Tsai WC, Chuang YC, Li CY, Wu TS, Kuoh CS, Chen WH, Chen HH (2008) A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X) 2–4 motif. Plant J 55:719–733
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Metabolic engineering of the indole pathway in Catharanthus roseus hairy roots and increased accumulation of tryptamine and serpentine. Metab Eng 6:268–276
Ikeda H, Esaki N, Nakai S, Hashimoto K, Uesato S, Soda K, Fujita T (1991) Acyclic monoterpene primary alcohol: NADP+ oxidoreductase of Rauwolfia serpentine cells: the key enzyme in biosynthesis of monoterpene alcohols. J Biochem 109:341–347
Irmler S, Schroder G, St-Pierre B, Crouch NP, Hotze M, Schmidt J, Strack D, Matern U, Schroder J (2000) Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24:797–804
Istvan ES, Deisenhofer J (2000) The structure of the catalytic portion of human HMG-CoA reductase. Biochim Biophys Acta 1529:9–18
Jacobs DI, Gaspari M, Van der Greef J, Van der Heijden R, Verpoorte R (2005) Proteome analysis of Catharanthus roseus cultured cells for the identification of proteins involved in alkaloid biosynthesis and finding of novel sequences. Planta 221:690–704
Kruczynsky A, Hill BT (2001) Vinflunine, the latest Vinca alkaloid in clinical development. A review of its preclinical anticancer properties. Crit Rev Oncol Hematol 40:159–173
Kutchan TM (2005) A role for intra- and intercellular translocation in natural product biosynthesis. Curr Opin Plant Biol 8:292–300
Kutchan TM (1998) Molecular genetics of plant alkaloid biosynthesis. In The Alkaloids. (Cordell G ed.) San Diego: Academic Press 50: 257–316
Kutney PJ, Hibino T, Jahngen E, Okutani T, Ratcliffe AH, Treasurywala AM, Wunderly SL (1976) Total synthesis of indole and dihydroindole alkaloids. IX. Studies on the synthesis of bisindole alkaloids in the vinblastine-vincristine series. The biogenetic approach. Helv Chim Acta 59(8):2858–2882
Kuzuyama T, Takagi M, Takahashi S, Seto H (2000) Cloning and characterization of 1-deoxy-D-xylulose 5-phosphate synthase from Streptomyces sp. strain CL190, which uses both the mevalonate and non mevalonate pathways for isopentenyl diphosphate biosynthesis. J Bacteriol 182:891–897
Laflamme P, St-Pierre B, De Luca V (2001) Molecular and biochemical analysis of a Madagascar periwinkle rootspecific minovincinine-19- hydroxy-O-acetyltransferase. Plant Physiol 125:189–198
Lange BM, Rujan T, Martin W, Croteau R (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci U S A 97:13172–13177
Langlois N, Gueritte F, Langlois Y, Potier P (1976) Application of a modification of the Polonovski reaction to the synthesis of vinblastine-type alkaloids. J Am Chem Soc 98:7017–7024
Learned RM, Connolly EL (1997) Light modulates the spatial patterns of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in Arabidopsis thaliana. Plant J 11:499–511
Lee S, Poulter CD (2006) Escherichia coli type I isopentenyl diphosphate isomerase: structural and catalytic roles for divalent metals. J Am Chem Soc 128(35):11545–11550
Lee M, Martin MN, Hudson AO, Lee J, Muhitch MJ, Leustek T (2005) Methionine and threonine synthesis are limited by homoserine availability and not the activity of homoserine kinase in Arabidopsis thaliana. Plant J 41:685–696
Levac D, Murata J, Kim WS, De Luca V (2008) Application of carborundum abrasion for investigating leaf epidermis: Molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J 53: 225–236
Li QR, Di Fiore S, Fischer R, Wang M (2003) Expression of tryptophan decarboxylase in chloroplasts of transgenic tobacco plants. Bot Bull Acad Sinica 44:193–198
Lichtenthaler HK, Rohmer M, Schwender J (1997) Two independent biochemical pathways for isopentenyl diphosphate (IPP) and isoprenoid biosynthesis in higher plants. Physiol Plant 101:643–652
Lois LM, Rodríguez-Concepcion M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J 22:503–513
Lopes Cardoso MI, Meijer AH, Rueb S, Queiroz Machado J, Memelink J, Hoge JHC (1997) A promoter region that controls basal and elicitor-inducible expression levels of the NADPH: cytochrome P450 reductase gene (Cpr) from Catharanthus roseus binds nuclear factor GT-1. Mol Gen Genet 256:674–681
Loyola-Vargas VM, Mendiz MZ, Miriam MG, De L, Mirinda-Ham M (1992) Serpentine accumulation during greening in normal and tumor tissues of Catharanthus roseus. Plant Physiol 140:213–217
Lu H, Gorman E, McKnight TD (2005) Molecular characterisation of two anthranilate synthase alpha subunit genes in Camptotheca acuminata. Planta 221:352–360
Luijendijk T (1995) Strictosidine glucosidase in indole alkaloid producing plants; characteristics and physiological implications. PhD thesis, Leiden University, The Netherlands
Luijendijk T, Stevens LH, Verpoorte R (1998) Purification and characterization of strictosidine b-D-glucosidase from Catharanthus roseus cell suspension cultures. Plant Physiol Biochem 36:419–425
Madyastha KM, Coscia CJ (1979) Detergent-solubilized NADPH-cytochrome c (P450) reductase from the higher plant, Catharanthus roseus. J Biol Chem 254:2419–2427
Mahroug S, Burlat V, St-Pierre B (2007) Cellular and sub-cellular organisation of the mono- terpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 6:363–381
Maldonado-Mendoza IE, Vincent RM, Nessler CL (1997) Molecular characterization of three differentially expressed members of the Camptotheca acuminata 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) gene family. Plant Mol Biol 34:781–790
Matthews PD, Wurtzel ET (2000) Metabolic engineering of carotenoid accumulation in Escherichia coli by modulation of the isoprenoid precursor pool with expression of deoxyxylulose phosphate synthase. Appl Microbiol Biotechnol 53:396–400
McFarlane J, Madyastha KM, Coscia CJ (1975) Regulation of secondary metabolism in higher plants. Effect of alkaloids on a cytochrome p450 dependent monooxygenase. Biochem Biophys Res Commun 66:1263–1269
McKnight TD, Bergey DR, Burnett RJ, Nessler CL (1991) Expression of enzymatically active and correctly targeted strictosidine synthase in transgenic tobacco plants. Planta 185:148–152
Meehan TD, Coscia CJ (1973) Hydroxylation of geraniol and nerol by a monooxygenase from Vinca rosea. Biochem Biophys Res Commun 53:1043–1048
Meijer AH, Verpoorte R, Hoge JHC (1993) Regulation of enzymes and genes involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Plant Res 3:145–164
Memelink J, Verpoorte R, Kijne JW (2001) ORC anisation of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Misra N, Gupta AK (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. J Plant Physiol 163:11–18
Misra N, Luthra R, Kumar S (2006) Bioconversion of Ajmalicine to Serpentine in Catharanthus roseus roots. J Plant Sci 1:340–347
Montamat F, Guilloton M, Karst F, Delrot S (1995) Isolation and characterization of a cDNA encoding Arabidopsis thaliana 3-hydroxy-3-methylglutaryl-coenzyme A synthase. Gene 167:197–201
Morant M, Bak S, Moller BL, Werck-Reichhart D (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol 14:151–162
Moreno PRH, Poulsen C, Heijden R, Verpoorte R (1996) Effects of elicitation on different metabolic pathways in Catharanthus roseus (L.) G. Don cell suspension cultures. Enzyme Microb Technol 18:99–107
Moreno PRH, Van der Heijden R, Verpoorte R (1993) Effect of terpenoid precursor feeding and elicitation of formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep 12:702–705
Moreno-Valenzuela OA, Minero-Garcia Y, Chan W, Mayer-Geraldo E, Carbajal E, Loyola-Vargas VM (2003) Increase in the indole alkaloid production and its excretion into culture medium by calcium antagonistic in Catharanthus roseus hairy roots. Biotechnol Lett 25:1345–1349
Morgan JA, Shanks JV (1999) Inhibitor studies of tabersonine metabolism in C. roseus hairy roots. Phytochemistry 51:61–68
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Mukherjee A, Basu S, Sarkar N, Ghosh A (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Murata J, De Luca V (2005) Localization of tabersonine 16-hydroxylase and 16-OH tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J 44:581–594
Murata J, Roepke J, Gordon H, De Luca V (2008) The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 20:524–554
Nejat N, Vadamalai G, Dickinson M (2012) Expression patterns of genes involved in the defense and stress response of Spiroplasma citri infected Madagascar Periwinkle Catharanthus roseus. Int J Mol Sci 13:2301–2313
Niyogi KK, Fink GR (1992) Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell 4(6):721–733
Ogura K, Koyama T (1998) Enzymatic aspects of isoprenoid chain elongation. Chem Rev 98:1263–1276
Osman MEH, Soad SE, Abo El-Soud K, Hasan AM (2008) Response of Catharanthus roseus shoots to salinity and drought in relation to vincristrine alkaloid content. Asian J Plant Sci 6:1223–1228
Oudin A, Courtois M, Rideau M, Clastre M (2007) The iridoid pathway in Catharanthus roseus alkaloid biosynthesis. Phytochem Rev 6:259–276
Page JE, Hause G, Raschke M, Gao W, Schmidt J, Zenk MH, Kutchan TM (2004) Functional analysis of the final steps of the 1-deoxy-D-xylulose (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiol 134:1401–1413
Papon N, Bremer J, Vansiri A, Andreu F, Rideau M, Creche J (2005) Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathway in Catharanthus roseus suspension cells. Planta Med 71:572–574
Pasquali G (1994) Regulation of the terpenoid indole alkaloid biosynthetic gene strictosidine synthase from Catharanthus roseus. PhD thesis, Leiden University
Peebles CA, Hughes EH, Shanks JV, San KY (2009) Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metab Eng 11:76–86
Phillips MA, D’Auria JC, Gershenzon J, Pichersky E (2008) The Arabidopsis thaliana type I isopentenyl diphosphate isomerases are targeted to multiple subcellular compartments and have overlapping functions in isoprenoid biosynthesis. Plant Cell 20:677–696
Pierre B, De Luca V (1995) A cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol 109:131–139
Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14:703–713
Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11:887–900
Poulsen C, Bongaerts R, Verpoorte R (1993) Purification and characterization of anthranilate synthase from Catharanthus roseus. Eur J Biochem 212:431–440
Powers R, Kurz WGW, De Luca V (1990) Purification and characterization of acetyl-CoA: deacetylvindoline 4-O-acetyltransferase from Catharanthus roseus. Arch Biochem Biophys 279:370–376
Proteau PJ (2004) 1-Deoxy-D-xylulose 5-phosphate reductoisomerase: an overview. Bioorg Chem 32:483–493
Querol J, Campos N, Imperial S, Boronat A, Rodríguez-Concepción M (2002) Functional analysis of the Arabidopsis thaliana GCPE protein involved in plastid isoprenoid biosynthesis. FEBS Lett 514:343–346
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91–102
Rai A, Smita SS, Singh AK, Shanker K, Nagegowda DA (2013) Heteromeric and homomeric geranyl diphosphate synthases from Catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol Plant 6(5):1531–1549
Rai V, Tandon PK, Khatoon S (2014) Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: vincristine and vinblastine. Biomed Res Int 2014, Article ID 934182
Ramos-Valdivia AC, van der Heijden R, Verpoorte R (1998) Isopentenyl diphosphate isomerase and prenyltransferase activities in rubiaceous and apocynaceous cultures. Phytochemistry 48:961–969
Richard SB, Ferrer JL, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP (2002) Structure and mechanism of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase—an enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. J Biol Chem 277:8667–8672
Rijhwani SK, Shanks JV (1998) Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Prog 14:442–449
Rodriguez S, Compagnon V, Crouch NP, St-Pierre B, De Luca V (2003) Jasmonate- induced epoxidation of tabersonine by cytochrome P-450 in hairy root cultures of Catharanthus roseus. Phytochemistry 64:401–409
Rodriguez-Concepcion M, Fores O, Martinez-Garcia JF, Gonzalez V, Phillips MA, Ferrer A, Boronat A (2004) Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell 16:144–156
Rohdich F, Wungsintaweekul J, Eisenreich W, Richter G, Schuhr CA, Hecht S, Zenk MH, Bacher A (2000) Biosynthesis of terpenoids: 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase of Arabidopsis thaliana. Proc Natl Acad Sci U S A 97:6451–6456
Rosco A, Pauli HH, Priesner W, Kutchan TM (1997) Cloning and heterologous expression of NADPH-cytochrome P450 reductases from the Papaveraceae. Arch Biochem Biophys 348:369–377
Ross IA (2003) Catharanthus roseus. Medicinal plants of the World, vol 1. Humana Press, Totowa, pp 175–196
Sanchez-Iturbe P, Galaz-Avalos RM, Loyola-Vargas VM (2005) Determination and partial purification of a monoterpene cyclase from Catharanthus roseus hairy roots. Phyton 55–69
Scheel D (1998) Resistance response physiology and signal transduction. Curr Opin Plant Biol 1(4):305–310
Schiel O, Witte L, Berlin J (1987) Geraniol-10-hydroxylase activity and its relation to monoterpene indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus. Z Naturforsch 42:1075–1081
Schmeller T, Wink M (1998) Utilization of alkaloids in modern medicine. In: Roberts MF, Wink M (eds) Alkaloids: biochemistry, ecology and medicinal applications. Plenum, New York, pp 435–459
Schmidt A, Gershenzon J (2008) Cloning and characterization of two different types of geranyl diphosphate synthases from Norway spruce (Picea abies). Phytochemistry 69:49–57
Schmidt A, Wachtler B, Temp U, Krekling T, Seguin A, Gershenzon J (2010) A bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Picea abies. Plant Physiol 152:639–655
Schroder G, Unterbusch E, Kaltenbach M, Schmidt J, Strack D, De Luca V, Schroder J, (1999) Light induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: tabersonine 16-hydroxylase. FEBS Lett 458: 97–102
Schulte AE, Vander Heijden R, Verpoorte R (2000) Purification and characterization of mevalonate kinase from suspension-cultured cells of Catharanthus roseus (L.) G. Don. Arch Biochem Biophys 378(2):287–298
Schwender J, Müller C, Zeidler J, Lichtenthaler HK (1999) Cloning and heterologous expression of a cDNA encoding 1-deoxy-D-xylulose-5-phosphate reductoisomerase of Arabidopsis thaliana. FEBS Lett 455:140–144
Seigler DS (1998) Plants with saponins and cardiac glycosides. www.lifwe.vinc.edu/plantbio/363/saponinslides
Shanks JV, Bhadra R (1997) Characteristics of selected hairy root lines of Catharanthus roseus. In: Doran PM (ed) Hairy roots. Harwood, Reading, pp 51–63
Sharma NK, Pan JJ, Poulter CD (2010) Type II isopentenyl diphosphate isomerase: probing the mechanism with alkyne/allene diphosphate substrate analogues. Biochemistry 49(29):6228–6233
Shet MS, Sathasivan K, Arlotto MA, Mehdy MC, Estabrook RW (1993) Purification, characterization, and cDNA cloning of an NADPH-cytochrome P450 reductase from mung bean. Proc Natl Acad Sci U S A 90:2890–2894
Sottomayor M, Lopez-Serrano M, Di Cosmo F, Ros-Barcelo A (1998) Purification and characterization of alpha-3,4-anhydrovinblastine synthase (peroxidase- like) from Catharanthus roseus (L.) G. Don. FEBS Lett 428:299–303
Stevens LH (1994) Formation and conversion of strictosidine in the biosynthesis of monoterpenoid indole and quinoline alkaloids. PhD thesis, Leiden University, The Netherlands
Stevens LH, Blom TJM, Verpoorte R (1993) Subcellular localization of tryptophan decarboxylase, strictosidine synthase and strictosidine glucosidase in suspension cultured cells of Catharanthus roseus and Tabernaemontana divaricata. Plant Cell Rep 12:563–576
St-Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of Catharanthus roseusalkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11:887–900
Tapsell LC, Hemphill I, Cobiac L (2006) Health benefits of herbs and spices: the past, the present, the future. Med J Aust 185:4–24
Tholl D, Lee S (2011) Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book 9:e0143, PMCID: PMC3268506
Treimer JF, Zenk MH (1979) Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation. Eur J Biochem 101:225–233
Uesato S, Matsuda S, Iida A, Inouye H, Zenk MH (1984) Intermediacy of 10-hydroxygeraniol, 10-hydroxynerol and iridodial in the biosynthesis of ajmaline and vomilenine in Rauwolfia serpentina suspension cultures. Chem Pharm Bull 32:3764–3767
Van der Heijden R, De Boer-Hup V, Verpoorte R, Duine JA (1994) Enzymes involved in the metabolism of 3-hydroxy-3- methylglutaryl coenzyme A in Catharanthus roseus. Plant Cell Tiss Org Cult 38:345–349
Van der Heijden R, Jacobs DI, Snoeijer W, Hallared D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:607–628
Vander Fits L, Memelink J (2000) ORCA3, a jasmonate responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Vander Fits L, Memelink J (2001) The jasmonate inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J 25:43–53
Vazquez-Flota FA, De Luca V (1998) Developmental and light regulation of desacetoxyvindoline 4-hydroxylase in Catharanthus roseus (L.) G. Don. Plant Physiol 117(4):1351–1361
Vazquez-Flota F, De Carolis E, Alarco AM, De Luca V (1997) Molecular cloning and characterization of desacetoxyvindoline 4-hydroxylase, a 2-oxoglutarate dependent dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol Biol 34:935–948
Veau B, Courtois M, Oudin A, Chenieux JC, Rideau M, Clastre M (2000) Cloning and expression of cDNAs encoding two enzymes of the MEP pathway in Catharanthus roseus. Biochim Biophys Acta 1517:159–163
Verpoorte R, Van der Heijden R, Memelink J (2000) Engineering the plant cell factory for secondary metabolite production. Transgenic Res 9:323–343
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Verpoorte R, Van der Heijden R, Moreno PRH (1997) Biosynthesis of terpenoid indole alkaloids in Catharanthus roseus cells. In: Cordell GA (ed) The alkaloids, vol 49. Academic Press, San Diego, pp 221–299
Vollack KU, Bach TJ (1996) Cloning of a cDNA encoding cytosolic acetoacetyl-coenzyme A thiolase from radish by functional expression in Saccharomyces cerevisiae. Plant Physiol 111:1097–1107
Wang G, Dixon RA (2009) Heterodimeric geranyl (geranyl) diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc Natl Acad Sci U S A 106:9914–9919
Wang DH, Du F, Liu HY, Liang ZS (2010) Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J Med Plants Res 4(24):2691–2699
Wang Q, Xing S, Pan Q, Yuan F, Zhao J, Tian Y, Chen Y, Wang G, Tang K (2012) Development of efficient Catharanthus roseus regeneration and transformation system using Agrobacterium tumefaciens and hypocotyls as explants. BMC Biotechnol 12:34–46
Whitmer S, van der Heijden R, Verpoorte R (2002a) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tissue Organ Cult 69:85–93
Whitmer S, Van der Heijden R, Verpoorte R (2002b) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase overexpressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Wolff M, Seemann M, Bui BTS, Frapart Y, Tritsch D, Estrabot AG, Rodriguez-Concepcion M, Boronat A, Marquet A, Rohmer M (2003) Isoprenoid biosynthesis via the methylerythritol phosphate pathway: the (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase (LytB/IspH) from Escherichia coli is a 4Fe-4S protein. FEBS Lett 541:115–120
Yamamoto H, Katano N, Ooi A, Inoue K (2000) Secologanin synthase which catalyzes the oxidative cleavage of loganin into secologanin is a cytochrome P450. Phytochemistry 53:7–12
Zhao J, Zhu W, Hu Q (2001a) Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Regul 33:43–49
Zhao J, Zhu W, Hu Q (2001b) Selection of fungal elicitors to increase indole alkaloid accumulation in Catharanthus roseus suspension cell culture. Enzyme Microb Technol 28(7):666–672
Zheng Z, Wu M (2004) Cadmium treatment enhances the production of alkaloid secondary metabolites in Catharanthus roseus. Plant Sci 166:507–514
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Akhtar, S., Ahmad, J., Ahmad, A. (2017). Molecular Network of Monoterpene Indole Alkaloids (MIAs) Signaling in Plants with Reference to Catharanthus roseus (L.) G. Don. In: Sarwat, M., Ahmad, A., Abdin, M., Ibrahim, M. (eds) Stress Signaling in Plants: Genomics and Proteomics Perspective, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-319-42183-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-42183-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42182-7
Online ISBN: 978-3-319-42183-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)