Abstract
Human pluripotent stem cells (hPSCs) present a potentially unlimited source of specialized cell types for regenerative medicine. Over the last few years there has been rapid progress in realizing this potential by developing protocols to generate disease-relevant cell types in vitro on demand. The approach was particularly successful for the nervous system, where the field is at the verge of human translation for several indications, including the treatment of eye disorders, Parkinson’s disease and spinal cord injury. More recently, there has also been success in deriving anterior pituitary lineages from both mouse and human pluripotent stem cells. In vitro-derived pituitary hormone-producing cell types present an attractive source for repair in patients with hypopituitarism. However, several hurdles remain towards realizing this goal. In particular, there is a need to further improve the efficiency and precision with which specific hormone-producing lineages can be derived. Furthermore, it will be important to assess the potential of both ectopic and orthotopic transplantation strategies to achieve meaningful hormone replacement. The ultimate challenge will be repair that moves beyond hormone replacement towards the full functional integration of the grafted cells into the complex regulatory endocrine network controlled by the human pituitary gland.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Derivation of Human Neural Cell Types for Regenerative Medicine
The isolation of human embryonic stem cells (ESCs; Thomson et al. 1998) and the remarkable feat of reprogramming somatic cells back to pluripotency via induced pluripotent stem cell (iPSC) technology (Takahashi et al. 2007; Takahashi and Yamanaka 2006; Yu et al. 2007) have set the stage for a new era of regenerative medicine. Human pluripotent stem cells (hPSCs), a term comprising both human ESCs and iPSCs, are characterized by their potential to differentiate into any cell lineage of the body. For many years, the main challenge in the field has been to capture the broad differentiation potential of hPSCs towards specific cell lineages relevant to modeling and treating human disease. However, there has been considerable progress recently in establishing differentiation protocol for many key lineages such as endoderm-derived insulin-producing pancreatic cells (Pagliuca et al. 2014; Rezania et al. 2014) for the treatment of diabetes or mesoderm-derived cardiac cells for heart repair (Chong et al. 2014). Some of the most dramatic successes, however, have involved ectoderm-derived lineages, in particular retinal and CNS lineages (for review see Tabar and Studer 2014)). In fact, the very first attempts at translating ESC technology towards the treatment of human patients was based on the use of oligodendrocyte precursor-like cells in patients with spinal cord injury (SCI: Alper 2009; Priest et al. 2015). However, SCI patients represent a challenging target for cell therapy, as the primary defect is a problem of connectivity between the brain and spinal cord rather than the loss of a specific cell type. Currently, the most widely pursued clinical target is the transplantation of hPSC-derived retinal pigment epithelial cells (RPEs) in patients with macular degeneration. There are nearly a dozen different RPE-based clinical trials either ongoing or in the planning phase (Kimbrel and Lanza 2015). Initial results using hESC-derived RPEs suggest that the approach can be translated safely into humans (Schwartz et al. 2015).
Beyond eye disorders, there has been particular interest in developing cell-based therapies for the treatment of various neurodegenerative disorders. In the case of Parkinson’s disease (PD), several studies demonstrated excellent in vivo survival of hPSC-derived midbrain dopamine (mDA) neurons in mouse, rat and non-human primate hosts (Kirkeby et al. 2012; Kriks et al. 2011). The transplantation of mDA neurons represents an example of replacing a highly specific neuronal subtype and a strategy that is thought to involve functional integration of the grafted cells into the existing neuronal networks. Indeed, a recent study from our group used optogenetics to demonstrate that functional rescue in the PD host animals depended on the continued neuronal activity of the grafted hESC-derived mDA neurons, and “switching-off” the graft led to a reversal of functional benefit within minutes (Steinbeck et al. 2015). The ability to derive mDA neurons from hESCs and hiPSCs and the promising pre-clinical data have set the stage for ongoing translational efforts towards testing this approach in human PD patients. Clinical trials are being planned in the US, Japan and Sweden, which have led to the formation of G-Force PD, a global effort to coordinate hPSC-based cell therapy efforts in PD (Barker et al. 2015). Another neurodegenerative disease being targeted is Huntington’s disease where several protocols have been published to generate authentic, striatal medium spiny neurons and where there is some initial evidence of efficacy in preclinical models (Arber et al. 2015; Delli Carri et al. 2013; Ma et al. 2012). Finally, several promising strategies are under development using glial cells. These include the transplantation of hPSC-derived oligodendrocytes in genetic models of white matter loss (Wang et al. 2013) and the remyelination of the brain following radiation-induced brain damage (Piao et al. 2015), a common and serious problem in cancer patients subjected to cranial irradiation (Greene-Schloesser et al. 2012; Schatz et al. 2000).
With our increasing ability to generate potentially any neural lineage on demand, the main challenge in the field has moved beyond making a specific cell type towards translation and therapy development in regenerative medicine. While the initial therapeutic targets for cell therapy are focused on replacing highly defined populations of cells such as RPEs or mDA neurons, it may be necessary in future studies to replace multiple cell types in combination to achieve meaningful rescue in a broader range of human disorders. A particular challenge for neuronal cell therapies is the importance of developing pre-clinical and ultimately clinical evidence that in vitro-derived cells can integrate into the complex circuitry of the human brain.
Derivation and Application of Human Pituitary Lineages
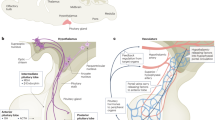
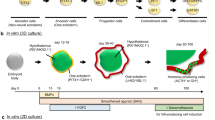
Replacing endocrine cells is conceptually more straightforward than replacing CNS neurons because there is no need to re-establish a complex synaptic circuitry to achieve improved function. However, the pituitary gland is also highly complex and acts as the master regulator of endocrine function, controlling a diverse range of responses in the body including stress control, growth and sexual function. Such complexity makes any treatment of hypopituitarism - the loss of pituitary function – challenging, as many hormones need to be replaced in a coordinated manner. In the context of cell therapy, this requires the ability to generate multiple hormone-producing cells at scale and on demand. To date, the main focus of hPSC-based approaches for treating endocrine disorders has been on the treatment of type I diabetes (Bruin et al. 2015). One key rationale for proposing a cell-based approach in diabetes is successful derivation of functional islet cells from hPSCs (Pagliuca et al. 2014; Rezania et al. 2014) and the expectation that grafted pancreatic β-cells will establish a feedback loop sensing glucose and adjusting insulin levels continuously throughout the day, something that is difficult to achieve by insulin injections. Furthermore, it appears likely that regulatory control can be achieved with cells that are not placed orthotopically into the pancreas but injected into a surgically more accessible tissue with high vascularity, such as the spleen or liver (Bruin et al. 2015). In contrast, orthotopic placement may be more critical for pituitary cells that respond to rapid and short acting signals from the hypothalamus. The challenge of recreating anterior pituitary lineage diversity in vitro was first tackled using mouse ESC cells. In a seminal study by the Sasai lab (Suga et al. 2011), a differentiation protocol was presented that allowed the derivation of mouse pituitary lineages via a serum-free embryoid body (SFEBq) culture step. SFEBq conditions were initially developed to generate forebrain lineages from mouse (Watanabe et al. 2005) and subsequently from human ESCs (Eiraku et al. 2008). In contrast to the forebrain, which is derived from the CNS, the anterior pituitary gland is derived from the oral ectoderm, which is part of the cranial placode lineages during development. To direct cell lineage towards oral ectoderm, Suga et al. (2011) showed that BMP4 exposure could trigger the induction of PITX2, an oral ectoderm marker, at the periphery of the differentiating SFEBs. Subsequent exposure to agonists of sonic hedgehog (SHH) signaling triggered expression of LHX3, which is a definitive anterior pituitary lineage marker. One remarkable feature during the induction process is the morphogenetic movements of the oral ectoderm that mimic the formation of Rathke’s pouch, an invagination of the oral ectoderm occurring during development that results in anterior pituitary gland formation. However, the overall efficiency of generating Pitx2+ oral ectoderm cells was low and most cells in the SFEBq cultures retained a neural identity. Approximately 1–7 % of the non-neural cells expressed specific hormones, a number that was dependent on further modulation of WNT activation for induction of growth hormone- (GH) or prolactin (PRL)-producing cells or inhibition of Notch signaling for obtaining ACTH+ cells (Suga et al. 2011). For the in vitro-derived ACTH+ cells, Suga et al. (2011) demonstrated CRH-dependent hormone secretion in vitro. Furthermore, transplanted cells were able to survive in vivo in an animal with surgically induced hypopituitarism, and they extended the life span of those animals, presumably by partially restoring their stress response. Some of the key questions raised by the Suga et al. study include whether the same technology can be applied for human ESC and iPSCs, whether the overall yield of anterior pituitary placode and hormone-producing cells can be improved and whether the 3D culture step, allowing the interaction of oral ectoderm-like and hypothalamic tissue, is critical for the efficient induction of anterior pituitary lineage cells.
Some initial answers to these questions came from an independent effort in our laboratories aimed at inducing cranial placode lineages from hPSCs (Dincer et al. 2013). Similar to the SFEBq technology, the human placode induction strategy was based on a protocol, dual-SMAD inhibition (Chambers et al. 2009), that was initially developed for inducing forebrain fates. Under dual-SMAD inhibition conditions, a monolayer of human ESCs or iPSCs can be converted at nearly 100 % efficiency into PAX6+ anterior neuroectoderm within about 10 days of differentiation (Chambers et al. 2009). Dual-SMAD inhibition involves concomitant exposure of hPSCs to inhibitors of BMP signaling (either Noggin or the ALK2/3 inhibitor LDN193189) and inhibitors of TGFβ, Activin and Nodal signaling (commonly via the small molecule compound SB431542). In Dincer et al. (2013), we showed that the key difference between CNS versus placode induction was the inhibition versus activation of BMP signaling. In contrast to the induction of CNS lineage under conditions of dual-SMAD inhibition, placode induction requires the timed removal of the BMP inhibitor at 48 h after neural induction, allowing endogenous BMP signaling to rebound. Under these default placode induction conditions, the majority of the hPSC-derived cells expressed PAX3, suggestive of trigeminal placode fate (Dincer et al. 2013). However, upon activation of SHH signaling, there was a marked increase in oral ectoderm markers such as PITX1 and SIX6. Further differentiation of these pituitary placode precursors was shown to yield various hormone-expressing cells, including ACTH+, FSH+ and GH+ lineages with clear evidence of in vitro hormone release. Finally, our study demonstrated that subcutaneous injection of hPSC-derived pituitary precursors into nude rats yielded measurable levels of ACTH and GH secretion in vivo. The findings of Dincer et al. suggested that the robust induction of human pituitary hormone-expressing cells did not require a 3D culture step. However, the overall efficiency of pituitary placode induction remained suboptimal. Furthermore, the media conditions during differentiation included components such as knockout serum-replacement (KSR) that are known to introduce batch-to-batch variability into the differentiation process. Furthermore, the study did not attempt to enrich for specific hormone lineages that may be required to develop better tailored therapies for each individual patient. A first step towards optimizing cranial placode induction in the absence of KSR was achieved by another study that carefully optimized the timing of BMP4 application (Leung et al. 2013). The results indicated that early exposure to BMP4 could increase overall cranial placode yield whereas the subsequent inhibition versus activation of BMP at later stages of differentiation could modulate the regional identity of hESC-derived placodal cells from PAX6+ anterior to PAX3+ posterior placode (Leung et al. 2013). Finally, the study confirmed that activation of SHH signaling increased the expression of oral ectoderm markers including PITX1 and PITX2.
More recently, members of the Sasai lab presented a study that adapted their 3D approach to human cells (Ozone et al. 2016). The study was based on a modified SFEBq culture system triggering differentiation in the presence of KSR, SHH agonist and BMP4 to yield 3D structures composed of hypothalamic cells in the center of the 3D aggregates and oral ectoderm cells at the periphery. In a proportion of those structures, the authors again observed the spontaneous formation of Rathke’s pouch-like structures similar to their original data in mouse ESCs, though at a lower frequency. While the overall induction efficiency of definitive pituitary lineages remained low, the authors were able to demonstrate both basal and CRH-induced release of ACTH in vitro that was shown to be suppressed by hydrocortisone treatment. Similarly, induction of GH could be modulated both positively and negatively by exposure to GHRH or somatostatin, respectively. Finally, subcutaneous injection of the 3D aggregates into mice with surgical hypophysectomy showed evidence of in vivo ACTH production that was responsive to CRH treatment. The transplanted cells also triggered significant, albeit very low, levels of corticosterone production and led to improved body weight and survival as compared to sham-grafted animals in hypophysectomized hosts. Some of the key remaining challenges include further improving the efficiency and reliability of pituitary lineage differentiation with a greater percentage of hormone-producing cells.
Perspectives and Challenges on the Road to Translation
The adenohypophysis (pituitary) is a remarkable endocrine organ that orchestrates the function of multiple targets via secretion of a set of regulatory hormones in charge of vital functions such as development, growth, puberty, reproduction, lactation and, crucially, response to stress. It receives regulatory endocrine input from the adjacent hypothalamus through a portal circulation system and communicates with the rest of the organism via an extensive network of vessels. Multi-tiered feedback is integrated by this master gland, leading to hormonal and metabolic homeostasis (Tabar 2011). The role of regenerative approaches to the adenohypophysis has received very little attention despite the prevalence of pituitary disorders and the large number of patients requiring pituitary hormone replacement due to traumatic brain injury, genetic, sporadic or iatrogenic disease. Several syndromes of pituitary deficiencies are recognized in humans as the result of mutations of early transcription factors or cell cycle regulator proteins (Melmed 2011). One of the prevalent causes of pituitary deficiency is post-treatment pituitary and hypothalamic damage. Specifically, and of interest to us, is a group of patients who suffer from hypopituitarism as a consequence of brain radiation for a variety of disorders, including hematological malignancies, head and neck cancers, brain tumors and sellar lesions (Appelman-Dijkstra et al. 2011). In fact, growing interest in cancer survivorship has identified hypopituitarism as a major contributor to poor quality of life indices (Darzy 2009). The clinical consequences are extensive and include fatigue, poor concentration, decreased memory and general cognitive abilities as well as significantly reduced well-being. In children these consequences are compounded by more serious learning difficulties and growth and skeletal problems, as well as a major impact on puberty and sexual function (Chemaitilly and Sklar 2010). Radiation damage to the hypothalamus and pituitary regions is progressive and irreversible. Current treatment consists of life-long multiple hormone replacement therapies, a suboptimal solution since static delivery of these molecules is a poor substitute for normal pituitary gland features such as the dynamic secretion of hormones in response to circadian patterns, feedback mechanisms or stressful conditions. In addition, treatment can be prohibitively expensive, with costs of growth hormone replacement alone exceeding $20,000 per year.
One of the key challenges to restorative strategies, regardless of the etiology of hypopituitarism, involves decisions regarding orthotopic or ectopic graft implantation. Grafts of pituitary tissue or primary cell suspensions from human fetal or rodent sources have been performed extensively using ectopic (Fu and Vankelecom 2012) or more or less orthotopic placement in the pituitary (Falconi and Rossi 1964), hypothalamus (Tulipan et al. 1985) or in the third ventricle (Vuillez et al. 1989). Overall, pituitary tissue or cells survive very well with the exception of conditions of immunological mismatch. A major concern with ectopic (i.e., subcutaneous or kidney) placement is the absence of hypothalamic control. The adenohypophysis is connected to the hypothalamus by a portal vein system that allows the immediate delivery of hypothalamic factors, thus bypassing the systemic circulation. Data from transplants in the hypothalamus, third ventricle or the hypophysis sites suggest that pituitary grafts demonstrate improved function and response to feedback when they are in immediate contact with the hypothalamus (Harris and Jacobsohn 1952; Maxwell et al. 1998). Some of the most successful results have been obtained upon transplantation in hypophysectomized female rats at the level of the median eminence, with good outcomes including restoration of estrus cycles, ability to conceive and lactate pups, adequate growth hormone and ACTH levels, as well as near normalization of body size in comparison to normal controls (Harris and Jacobsohn 1952). Data from transplantation of mouse ESC-derived pituitary cells (injected in the renal capsule) suggested statistically significant elevation of basal ACTH and corticosterone (Suga et al. 2011). From a translational perspective, ectopic placement in the subcutaneous tissue offers significant advantages due to the low risk of the intervention (e.g., in a subcutaneous location) and easy accessibility in case of complications. The experimental evaluation of ectopic grafts may therefore be a justifiable strategy, though integration within the hypothalamic-pituitary-target organ axis and homeostatic control is more likely to be achieved if grafts are placed in the vicinity of the hypothalamus or even within the gland itself. Interestingly, in humans, placement in the sella is simpler than in rodents, due to the development of minimal invasive transnasal endoscopic approaches to the sella and anterior skull base. Experimental evidence of hypothalamic control upon grafting of pituitary cells requires complex assays and readouts, including stimulation tests [e.g., response to thyrotropin releasing hormone (TSH) or to growth hormone-releasing hormone (GHRH) etc.], physiological stress tests [e.g., exposure to cold, arginine testing followed by evaluation of variations in pituitary hormone levels (Akalan et al. 1988; Fisker et al. 1999; Guillemin 2005)]. Behavioral testing can also contribute to the evaluation of the integrity of the hypothalamic-pituitary-target organ axis and its feedback loops. Demonstrating appropriate integration into the neuroendocrine system and its physiological and homeostatic feedback loops should be considered an important component of both efficacy and safety of this strategy. Obviously uncontrolled or random secretion of key hormones such as ACTH or growth hormone can have serious negative health consequences.
Additional considerations in translational strategies would include the possibility of grafting specific pituitary sublineages, e.g., ACTH- or growth hormone-secreting. This approach might require more sophisticated differentiation protocols and, likely, selection strategies that should be compatible with good manufacturing practice (GMP) conditions and safety standards.
Conclusion
The recent successful derivation of pituitary placode lineage and the range of anterior pituitary hormone-producing cells are very exciting advances that will likely herald the development of restorative strategies in humans. Several challenges along the road to translation remain to be tackled. Key questions include the ability to develop selective pituitary sublineages that produce a single target hormone, the development of grafting strategies for human patients, and the demonstration of integration of the grafted cells into the hypothalamic-pituitary-peripheral target axis, a goal that is fundamental to the safety assessment of the cell therapy-based approach to hypopituitarism.
Note Added in Proof
A recent manuscript from our team presents a fully defined and efficient protocol for the derivation of anterior pituitary hormone producing cells from human pluripotent stem cells and demonstrates hormone release in a rat model of hypopituitarism (Zimm et al. 2016).
References
Akalan N, Pamir MN, Benli K, Erbengi A, Erbengi T (1988) Fetal pituitary transplants into the hypothalamic area of hypophysectomized rats. Surg Neurol 30:342–349
Alper J (2009) Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol 27:213–214
Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, Neelis KJ, Biermasz NR, Romijn JA, Smit JW, Pereira AM (2011) Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metab 96:2330–2340
Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodriguez TA, Rosser AE, Dunnett SB, Li M (2015) Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 142:1375–1386
Barker RA, Studer L, Cattaneo E, Takahashi J (2015) G-Force PD: a global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. Npj Parkinson’s Dis 15017. doi:10.1038/npjparkd.2015.17
Bruin JE, Rezania A, Kieffer TJ (2015) Replacing and safeguarding pancreatic beta cells for diabetes. Sci Translat Med 316ps323
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280
Chemaitilly W, Sklar CA (2010) Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer 17:R141–159
Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510:273–277
Darzy KH (2009) Radiation-induced hypopituitarism after cancer therapy: who, how and when to test. Nat Clin Pract Endocrinol Metab 5:88–99
Delli Carri A, Onorati M, Lelos MJ, Castiglioni V, Faedo A, Menon R, Camnasio S, Vuono R, Spaiardi P, Talpo F, Toselli M, Martino G, Barker RA, Dunnett SB, Biella G, Cattaneo E (2013) Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development 140:301–312
Dincer Z, Piao J, Niu L, Ganat Y, Kriks S, Zimmer B, Shi SH, Tabar V, Studer L (2013) Specification of functional cranial placode derivatives from human pluripotent stem cells. Cell Rep 5:1387–1402
Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532
Falconi G, Rossi GL (1964) Method for placing a pituitary graft into the evacuated pituitary capsule of the hypophysectomized rat or mouse. Endocrinology 75:964–967
Fisker S, Nielsen S, Ebdrup L, Bech JN, Christiansen JS, Pedersen B, Jorgensen JO (1999) The role of nitric oxide in l-arginine-stimulated growth hormone release. J Endocrinol Invest 22:89–93
Fu Q, Vankelecom H (2012) Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev 21:3245–3257
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Guillemin R (2005) Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol 184:11–28
Harris GW, Jacobsohn D (1952) Functional grafts of the anterior pituitary gland. Proc R Soc Lond Ser B Biol Sci 139:263–276
Kimbrel EA, Lanza R (2015) Current status of pluripotent stem cells: moving the first therapies to the clinic. Nat Rev Drug Discov 14:681–692
Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M (2012) Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 1:703–714
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480:547–551
Leung AW, Kent Morest D, Li JY (2013) Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells. Dev Biol 379:208–220
Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC (2012) Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10:455–464
Maxwell M, Allegra C, MacGillivray J, Hsu DW, Hedley-Whyte ET, Riskind P, Madsen JR, Black PM (1998) Functional transplantation of the rat pituitary gland. Neurosurgery 43:1157–1163
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266
Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y (2016) Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun 7:10351
Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA (2014) Generation of functional human pancreatic beta cells in vitro. Cell 159:428–439
Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, Tabar V (2015) Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell 16:198–210
Priest CA, Manley NC, Denham J, Wirth ED 3rd, Lebkowski JS (2015) Preclinical safety of human embryonic stem cell-derived oligodendrocyte progenitors supporting clinical trials in spinal cord injury. Regen Med 10:939–958
Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ (2014) Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32:1121–1133
Schatz J, Kramer JH, Ablin A, Matthay KK (2000) Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology 14:189–200
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385:509–516
Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L (2015) Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson's disease model. Nat Biotechnol 33:204–209
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480:57–62
Tabar V (2011) Making a pituitary gland in a dish. Cell Stem Cell 9:490–491
Tabar V, Studer L (2014) Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15:82–92
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Tulipan NB, Zacur HA, Allen GS (1985) Pituitary transplantation: Part 1. Successful reconstitution of pituitary-dependent hormone levels. Neurosurgery 16:331–335
Vuillez P, Moos F, Stoeckel ME (1989) Immunocytochemical and ultrastructural studies on allografts of the pituitary neurointermediate lobe in the third cerebral ventricle of the rat. Cell Tissue Res 255:393–404
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA (2013) Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12:252–264
Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8:288–296
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zimmer B, Piao J, Ramnarine K, Tomishima MJ, Tabar V, Studer L (2016) Derivation of diverse hormone-releasing pituitary cells from human pluripotent stem cells. Stem Cell Rep 6(6):858–872. doi:10.1016/j.stemcr.2016.05.005, PMID: 27304916
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, a link is provided to the Creative Commons license and any changes made are indicated.
The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Studer, L., Tabar, V. (2016). Human Pluripotent-Derived Lineages for Repairing Hypopituitarism. In: Pfaff, D., Christen, Y. (eds) Stem Cells in Neuroendocrinology. Research and Perspectives in Endocrine Interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-41603-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-41603-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41602-1
Online ISBN: 978-3-319-41603-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)