Abstract
A simple protocol is presented that tests salt tolerance in rice seedlings. The method is based on a glasshouse hydroponics test in which salt is added to the nutrient hydroponic solution in which the seedlings are grown. A list of equipment is provided including hydroponic hardware and stock solutions. Advice is given on seed storage prior to use and pregermination treatments that promote even germination of test samples. Salt treatments commence after seedling establishment in hydroponics, at the 2–3 leaf stage. Information on responses of standard genotypes (tolerant, intermediate and sensitive) is given to which test seedlings are compared. Visual symptoms of salinity stress include reduced leaf area, whitish appearance of lower leaves, leaf tip death, leaf rolling and seedling death. Scoring is carried out according to the standard evaluation system developed by the International Rice Research Institute (IRRI). Recommended test salt concentrations are given along with a method to recover selected seedlings and examples of use.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Rice is one of the most important crops and is consumed by more than half of the world’s population. Soil salinity is a major and increasing problem limiting rice growth and leads to huge yield losses every year. The search for new cultivars with improved tolerance to salt stress is a major goal in relieving this problem. This protocol gives an easy-to-follow procedure to select salt-tolerant rice lines for subsequent field testing.
4.2 Equipment
All equipment (tanks, trays, containers, drums and platforms) is dark coloured to minimise light penetration into the culture solution, thus reducing algal growth.
-
Test tanks: These are made of plastic and have outside dimensions of 60 × 40 × 12 cm and contain approximately 24 l each when full (Fig. 4.1). The size of tank can be changed to suit local conditions.
-
Recovery tanks: These are made of plastic with outside measurements of 40 × 30 × 17 cm. These hold approximately 20 l.
-
Germination lids: PVC covers are used to blank out light; these sit over the PVC support platforms to provide darkness during germination (not obligatory). Germination lid dimensions: 50 × 34 × 2 cm (Fig. 4.1b). The lids promote germination by helping to maintain humidity and temperature and cut out light.
-
Support platforms:
-
1.
M2 test platforms: PVC support platforms are made up with the dimensions 56 × 36 × 1.2 cm to fit inside the top of a test tank. These platforms overlap the top of the test tank by 2 cm by gluing an additional sheet of PVC (5 × 36 × 1.2 cm) at both ends (Fig. 4.1a). M2 screening platforms contain 24 rectangular compartments (6 × 7 cm) cut at regular intervals with a spacing of 1.2 cm. Each compartment can accommodate 100–200 seeds (useful for M2 screening). Nylon mesh (fly netting) is cut to fit the PVC platforms (56 × 36 cm) and glued to the underside using PVC-V glue.
-
2.
M3 and other advanced generation/line test platforms: These PVC support platforms are made up with the dimensions 36.5 × 26.5 × 1.2 cm. These overlap the test tanks by 2 cm by fitting an addition sheet of PVC (5 × 36 × 1.2 cm) at both ends (Fig. 4.1c). Round holes are drilled out (100 round holes, 2 cm diameter). Two of these support platforms can sit on top of one big test tank.
-
3.
Support platforms for recovery tanks: PVC platforms are made up with the dimensions 36.5 × 26.5 × 1.2 cm. These overlap the tanks by 2 cm by fitting an addition sheet of PVC (5 × 36 × 1.2 cm) at both ends (Fig. 4.1c, d). Thirty equidistant open holes (2.2 cm diameter) are drilled into the support platforms (without mesh).
-
1.
-
Sponge strips (10 × 2 × 1 cm) (Fig. 4.1d).
-
Storage containers for stock solutions: Stock solution can be prepared in small amounts and stored in the glasshouse or at room temperature for 1–2 months; mineral precipitation or the change in the covalence such as Fe or Cu in the solution is negligible over this period. The storage containers are air- and lighttight to allow long storage (1–2 months, Fig. 4.2). Nutrients for rice hydroponics have been described by Yoshida et al. (1976) and consist of six stock solutions (five for major elements and a sixth one for all microelements); for convenience, these are normally made up in 5 l amounts (Table 4.1).
Table 4.1 Constitution of stock solutions -
Storage drums for the working Yoshida solution: The working solution is made up using the six stock solutions and then diluted with distilled water in large drums. For convenience, the drum may be fitted with a submersible water pump to aid mixing, aeration and distribution into tanks. The solution may be prepared fresh or stored for incorporation in the next pH and volume adjustment (every 2 days). Large volumes of Yoshida solution (up to 120 l) may be stored in airtight and lighttight drums in the glasshouse for up to 1 week.
-
pH meter.
-
Electrical conductivity meter.
Note: Distilled water is preferred in making up Yoshida solution as local tap water may result in precipitation of minerals and will alter mineral concentrations that may affect salt sensitivity.
4.3 Plant Materials
Test materials should be compared against standard genotypes of known salt tolerance. Standards used at the Plant Breeding and Genetics Laboratory (PBGL), Seibersdorf, Austria, are as follows:
-
Pokkali: Salt-tolerant wild type
-
Nona Bokra: Salt-tolerant wild type
-
Bicol: Moderately salt tolerant
-
STDV: Moderately salt tolerant (induced mutant from IR29)
-
Taipei 309: Salt susceptible
-
IR29: Salt susceptible
The salt tolerance of the above standards in saline hydroponics has been correlated with the field performance (Gregorio et al. 1997; Afza et al. 1999). These standard materials can be requested free of charge from IRRI under a Standard Materials Transfer Agreement. Alternatively, local cultivars or breeding lines of known salt tolerance may be used as standards.
4.4 Setting Up Hydroponic Hardware
The screening is done in glasshouse conditions with day/night temperatures of 30/20 °C and relative humidity of at least 50 % during the day. The glasshouse should be disease free and well lit by natural or artificial lighting. The tanks may be placed on the floor or on the bench, but the surface should be as levelled as possible; tank water levels may also be adjusted using wedges.
4.5 Preparation of Hydroponic Solutions
The working solution is prepared as described by Yoshida et al. (1976) with adaptations made by Gregorio et al. (1997) (Table 4.2): each stock solution is shaken and 150 ml samples of each stock are mixed together and made up to 120 l. The pH of the working solution is adjusted in the drum to 5.0 with 1 N sodium hydroxide (NaOH) and 1 N hydrochloric acid (HCl) with continuous stirring (a pump may be used) to insure the solution is homogenised; this simultaneously aerates the solution.
4.6 Seed Storage and Seed Pregermination Treatments
Seed should be stored in dry, airtight containers at 4 °C. Germination of seed should be determined before testing as it is essential that seeds germinate uniformly (at the same rate) and that sufficient seedlings are available for testing. Some seed samples may have high seed dormancy; this can be broken by heat treatment at 40–50 °C for 2–5 days. Seed samples may also suffer from varying degrees of microbial contamination. This can be controlled by surface sterilisation by soaking in 0.8 % sodium hypochlorite (NaClO) for 20 min and then washing three times with water. Solutions of sodium hypochlorite can be easily made from commercial bleach (about 5 % NaClO). This treatment also promotes even germination.
4.7 Seedling Establishment in Hydroponics
The tests are normally conducted in a glasshouse set-up for rice: 30/20 °C day/night temperatures with 70 % humidity and 16 h photoperiod. Test tanks are filled with distilled water until the water level is about 1 mm above the mesh. The water level may be adjusted using wedges. Seeds are then placed into the wet compartments. For M2 screening, 30–50 seeds from one panicle are placed into one compartment (6 × 7 cm) (Fig. 4.3a); for M3 and advanced line testing, 5–10 seeds (or germinated seeds) are placed into each 2 cm diameter compartment; lines may be replicated within and among tanks (Fig. 4.3b). The test platforms are then covered with a lid for 1 week to promote germination in the dark. At day three, the water is replaced with half-strength Yoshida solution as vigorously growing seedlings will require nutrients. After 1 week, the platform of germinated seed is transferred to a test tank containing full-strength Yoshida solution to establish healthy seedlings prior to salt treatment. Seedlings are grown on to the two-leaf stage and should appear green and healthy prior to testing.
(a) M2 platform with rice seeds ready for germination; each compartment contains seed from one panicle per M1 plant. (b) M3/advanced line platform showing rice seed ready for germination; each compartment contains 3–5 seeds from each line. (c) Rice seedlings at the 2–3 leaf stage ready for salt screening. (d) Rice seedlings showing various degrees of salt injury
Note: The test should not be carried out on unhealthy seedlings.
Note: If seed samples are not clean and rotting occurs during germination, these must be removed. Seed may be surface sterilised prior to germination by soaking in 20 % Clorox solution for 20–30 min, followed by three rinses in distilled water. Clorox treatment also helps to promote germination.
4.8 Care of Plants in Hydroponics
Due to evaporation and transpiration, there will be loss of solution volume and pH change (algal growth may also contribute in pH fluctuation). Every 2 days (or thrice a week), the volume needs to be brought back to the level of full capacity (touching the netting in the platform compartments) and the pH adjusted to 5. Solutions can be changed by lifting off the platforms and placing them temporarily onto empty tanks and pouring the hydroponic solutions back into a drum where the bulked solution can be pH adjusted for the whole experiment in one step. Once adjusted, the solution is redistributed into the test tanks and the seedling platform returned. These operations also act to aerate the hydroponic solution. Alternatively, the pH can be adjusted on an individual tank basis, and more working solution may be added to make up the volume in each tank.
4.9 Salt Treatment
Salt treatment is applied at the 2–3 leaf stage, after 1–2 weeks of seedling establishment in full-strength Yoshida solution (depending on the rate of seedling establishment, Fig. 4.3c). The salt treatment is applied in one go and not incrementally. The test salt concentration is 10 dS/m (10 dS/m corresponds to 4.8 and 6.4 g of NaCl, respectively, in 1 l Yoshida solution and distilled water). Table 4.3 provides the conversion of NaCl in g/L in Yoshida solution for mmol and dS/m. Salinisation of the nutrient solution (working solution) is done for large volumes by adding dry NaCl in a drum containing Yoshida, dissolved and mixed using a submersible water pump. Salt is added until the 10 dS/m is reached; electrical conductivity is measured using an electrical conductivity meter (EC meter).
4.10 Scoring
Visual symptoms of salinity stress are reduced leaf area, whitish appearance of lower leaves, leaf tip death and leaf rolling. The technique for salinity screening is based on the ability of seedlings to grow in salinised nutrient solution. Standard genotypes are normally included in each test tank for comparison. Scoring is relative and carried out according to the standard evaluation system developed by IRRI with a score 1 for tolerant and 9 for sensitive (Table 4.4). Scoring is carried out at or around day 12 of salt treatment. At this stage, sensitive seedlings begin to die, whereas intermediate genotypes show varying degrees of tolerance (Fig. 4.3d). Table 4.5 gives classification criteria for salt tolerance based on known standards.
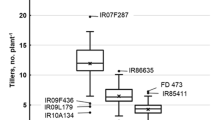
Note: Scoring may be carried out at each day of treatment if quantitative data are required. Growth curves may be plotted to study responses over time. The biomass of seedlings may be recorded for this purpose using shoot/root/whole plant weight (fresh and dry), plant height and tillering that can be scored during the qualitative evaluation over time. However, scoring should be carried out for longer than 12 days of salt treatment as it is at this point that growth reduction of susceptible seedling becomes most apparent, whereas tolerant seedlings show some growth increase (but reduced compared to control seedlings).
At day 12 of salt treatment, tolerant standards (Pokkali and Nona Bokra) show slight damage with leaf tips becoming brown; moderately tolerant standards (Bicol and STDV) exhibit more leaf damage with dead older leaves and younger leaves being green only at their leaf bases; susceptible standards (IR29 and Taipei 309) are dead.
Susceptible lines will die at the same time as or before the sensitive standard IR29.
Moderately tolerant lines will respond in a similar manner to Bicol.
Tolerant lines can be selected when Bicol begins to die or has died; these may be removed to a recovery tank.
Symptoms on selected tolerant lines may be compared to Pokkali to estimate the degree of tolerance.
In cases where no standard lines are available, the following table may be used to assess tolerance in seedlings (Table 4.5). This table has been adapted from “Screening rice for salinity tolerance” (Gregorio et al. 1997).
4.11 Recovery of Salt-Tolerant Lines
Selected tolerant seedlings are teased out of the test tanks with care taken to keep roots intact. The base of the aerial part of each selected seedling is then gently wrapped in a sponge strip (10 × 2 × 1 cm) and the seedlings inserted into a recovery tank (Fig. 4.1d).
Selected seedlings can be grown to maturity in these tanks filled with Yoshida solution changed every 2 weeks.
4.12 Examples
The following tables summarise salinity data from results of seedling hydroponics screening carried out at the PBGL on materials from Iran (Table 4.6), Myanmar (Table 4.7) and Vietnam (Table 4.8).
References
Afza R, Zapata-arias FJ, Zwiletitsch F, Berthold G, Gregorio G (1999) Modification of a rapid screening method of rice mutants for NaCl tolerance using liquid culture. Mutat Breed Newsl 44:25–28
Gregorio GB, Senadhira D, Mendoza RT (1997) Screening rice for salinity tolerance. IRRI Discussion Paper series 22, vol 22. IRRI, Manila, p 30
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice. IRRI, Las Banos, Laguna, p 83
Author information
Authors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2016 International Atomic Energy Agency
About this chapter
Cite this chapter
Bado, S., Forster, B.P., Ghanim, A.M.A., Jankowicz-Cieslak, J., Berthold, G., Luxiang, L. (2016). Protocol for Screening for Salt Tolerance in Rice. In: Protocols for Pre-Field Screening of Mutants for Salt Tolerance in Rice, Wheat and Barley. Springer, Cham. https://doi.org/10.1007/978-3-319-26590-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-26590-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26588-9
Online ISBN: 978-3-319-26590-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)