Abstract
Fifty-nine diverse Brassica juncea (Indian mustard) genotypes were used to find an effective screening method to identify salt tolerance at the germination and seedling stages. Salinity stress limits crop productivity and is difficult to simulate on farms, hindering parental selection for hybridization programmes and the development of tolerant cultivars. To estimate an optimum salt concentration for screening, seeds of 15 genotypes were selected randomly and grown in vitro at 0 mM/L, 75 mM/L, 150 mM/L, 225 mM/L, and 300 mM/L concentrations of NaCl in 2 replications in a complete randomized design. Various morphological parameters, viz., length of seedling, root and shoot length, fresh weight, and dry weight, were observed to determine a single concentration using the Salt Injury Index. Then, this optimum concentration (225 mM/L) was used to assess the salt tolerance of all the 59 genotypes in 4 replications while observing the same morphological parameters. With the help of Mean Membership Function Value evaluation criteria, the genotypes were categorized into 5 grades: 4 highly salt-tolerant (HST), 6 salt-tolerant (ST), 19 moderately salt-tolerant (MST), 21 salt-sensitive (SS), and 9 highly salt-sensitive (HSS). Seedling fresh weight (SFW) at 225 mM/L was found to be an ideal trait, which demonstrates the extent to which B. juncea genotypes respond to saline conditions. This is the first report that establishes a highly efficient and reliable method for evaluating the salinity tolerance of Indian mustard at the seedling stage and will facilitate breeders in the development of salt-tolerant cultivars.
Similar content being viewed by others
Introduction
Several abiotic stresses, such as extreme temperatures, drought, floods and soil salinity, have negatively impacted the growth and cultivation of agricultural crops. Among these, soil salinity is particularly destructive, leading to significant decreases in arable land, crop yield, and quality1,2,3. In coastal areas, salinity is caused by ocean water influx, whereas inland salinity is caused by inadequate irrigation practices and high salt deposition in ground water4,5,6.Salinity affects around 33% of irrigated agricultural fields and 20% of all cultivated agricultural fields globally and such regions are increasing at an alarming rate of 10% every year due to a variety of factors such as weathering of rocks, low precipitation, irrigation with saline water, excessive surface evaporation, and inefficient cultural practices7,8. Soil salinity impacts crop physiology along with various molecular and biochemical functions. It affects seed germination and seedling growth while inhibiting root, shoot elongation and dry matter accumulation9,10,11. Osmotic stress is the first stress a plant encounters when exposed to saline conditions which immediately slows down the rate of germination and subsequent plant growth12,13,14. Excessive salinity has negative effects on plant photosynthetic activities, causing leaf injury, chlorosis, and senescence15. Due to the high concentration of soluble salts in the soil, such as Ca2+, Mg2+, Na+, and Cl–, soil water availability decreases causing nutritional imbalance and production of toxic ions affecting the plant growth16,17. Moreover, plants affected by salt stress are more susceptible to disease outbreaks2. Several comprehensive analyses on techniques for addressing the salinity issue highlighted two main approaches: the reclamation of salt-affected soils through the application of chemical additives, or the use of saline soils for cultivating salt-tolerant plants18,19,20,21. The latter approach is more practical considering demonstrated availability of adapted genetic variation in various species.
Brassicas are the third-most important edible oil crop in the world, ranked after soybean and palm oil22. Higher salt tolerance is exhibited by amphidiploid Brassica species like B. carinata, B. juncea, and B. napus compared to their diploid progenitors, B. oleracea, B. nigra, and B. rapa23. Among them, B. juncea also known as Indian mustard was grown extensively in India and China until the late 1950s, when B. napus became more productive. New to Australia (1980s) and Canada (2002), it has substantial morphological and physiological heterogeneity24 and stands out as one of the most essential oil-yielding crops in salinity affected soils25. Given that the salt tolerance level in B. juncea remains consistent throughout its entire life cycle, selecting for salt tolerance during the earliest development stages might yield individuals which are tolerant at all subsequent growth phases19,26. Earlier studies have used various morphological traits of seedlings as well as biochemical and physiological traits ranging from levels of osmoprotectants, K+/Na+ ratio, total osmolytes, photosynthetic rate, water use efficiency rate and total chlorophyll content to assess salinity tolerance27,28,29,30,31,32. In recent times, morpho-physiological traits have been observed at the germination stage not only with the aim of screening salt tolerance but also to determine a reliable trait or indicator in different species. These studies have utilized a mathematical approach to screen for salinity stress known as Fuzzy set theory, it provides a framework for capturing the gradual and multifaceted nature of plant response to stress enabling a more nuanced understanding of complex traits as salinity and drought tolerance33. Root shoot length and shoot fresh weight have been reported as indicators for salt tolerance in B. napus34,35, germination index along with germination vigour index in sunflower36, seedling vigour index in rice37 and root length in chickpea38. Multiple studies exist that explore salinity tolerance in Indian mustard; however, there is a noticeable gap in the literature concerning the identification of a single reliable trait for assessing salinity tolerance in Brassica juncea during the germination stage39,40,41,42,43. It is crucial to identify an effective and reliable trait for the assessment and screening of salt-tolerant lines for breeding salt tolerant varieties. It would be one step forward for breeders to screen large number of germplasm at the seedling stage itself and thus in this study, we investigate the salt tolerance of 59 B. juncea genotypes at different salinity levels to establish an effective salt concentration at which the germplasm can be screened for salt stress tolerance and a reliable screening parameter.
Methods
Experimental material
There were two main objectives of this study, first to determine the optimum salt concentration for screening salt tolerance and second to determine a reliable screening trait for salinity tolerance at the germination/seedling stage. For this purpose, seeds of 59 Brassica juncea genotypes were obtained from Lovely Professional University, these are a set of diverse released genotypes adapted to Indian sub-continent conditions. The experiment was conducted in 2023 using a completely randomized design (CRD) at the Lovely Professional University Plant Breeding Laboratory, wherein seeds were grown in vitro in petri plates in a growth chamber while maintaining a temperature of 28 °C during the day and 23 °C at night with humidity ranging from 50 to 70%.
Determination of an optimum NaCl concentration
Initially, to establish the ideal salt concentration, a total of fifteen genotypes were chosen at random on which four different salt concentrations were used35,36. The control treatment in this experiment consisted of distilled water (0 mM/L) and a range of four different NaCl concentrations (75, 150, 225, and 300 mM/L) were utilised. A selection of twenty seeds was made from each genotype, ensuring that they displayed uniformity and good health. These seeds were subjected to surface sterilisation using 15 mL of 70% ethanol for a duration of 15 min. Following this, they were rinsed thoroughly using distilled water. The seeds were then immersed in pure water for a period of 12 h. These seeds were then evenly distributed in petri plates using two layers of blotting paper as base. Seeds from two genotypes with the same NaCl concentrations were placed on either side of each petridish, which was divided into two halves using a thread. Each petri dish was then filled with 12 mL of either distilled water (control) or one of the aforementioned NaCl solutions. To prevent evaporation, the petri dishes were covered with a lid and kept in growth chamber with a constant 12 h of light and dark periods, at a temperature of 28 °C during the day and 23 °C at night. Two biological replicates were used in this stage of the experiment. Each petri dish was examined every two days, and using a dropper, the old solution was replaced with an equal amount of the new solution. In the control treatment, the same procedure was used to replace the old distilled water with new. When the length of a seed's radicle reached or went over 2 mm, it was considered that the seed had germinated. Various morphological parameters like rate of germination, root length, shoot length, seedling length, seedling fresh weight and dry weight were measured as mentioned below. The appropriate salt concentration was determined by assessing the salt-injury index (SII) for each parameter, which was regarded to be 0.5 to that of control. In other words the salt concentration was considered to be the best-fit concentration when the salt injury index (SII) was 50% of the control because at this point the seedlings begin to show the extent of damage or injury caused by salt stress on a plant35.
Rate of germination (G) was calculated using following formula36;
where \({\text{G}}_{\text{t}}=\text{Number \, of \, seeds \, germinated }7\text{ DAS},\text{ T}=\text{Total \, seeds \, sown}, \)(DAS = days after sowing/placing).
Measurements for Lengths of root (RL), shoot (SHL) and seedling (SL) were taken in centimetres and for Fresh weight of seedling (SFW) in milligrams after 7 DAS.
Measurements for Dry weight of seedling (SDW) were taken individually in milligrams following uniform drying at 70 °C until a constant weight was attained.
Salt Injury Index (SII) and Salt Tolerance Index (STI) were calculated as follows:
STI was calculated for each parameter to eliminate the effect of genotypic background34,35.
Determination of tolerance at optimum salt concentration
The salt tolerance of all 59 B. juncea genotypes was determined using the optimum salt concentration (225 mM/L) determined in first experiment. Ten healthy seeds of the same genotype were placed in each half of each 9 cm petri dish, which was divided into two equal portions and treated with the 225 mM/L NaCl solution (Fig. 1). A separate control treatment with a concentration of 0 mM/L was also maintained. The biological replicates of each genotype were four. Each of the four replications, as well as the control, contained ten seeds each. A similar sterilisation procedure was carried out before administering NaCl solutions, as previously mentioned. The petri dishes were monitored on a regular basis, and old solution was replaced with new solution. To evaluate salt tolerance of all B. juncea genotypes, same morphological parameters as mentioned above were determined.
The estimation of salt tolerance levels was conducted by utilising the fuzzy comprehensive evaluation approach, which relied on the membership function value44. The MFV was determined as follows:
where \({\text{X}}_{\text{i }}=\text{MFV \, of \, a \, trait},\text{ X}=\text{STI \, of \, a \, trait}, {\text{X}}_{\text{max}}=\text{Maximum \, STI \, value}, {\text{X}}_{\text{min}}=\text{Minimum \, STI \, value}\)
Mean MFV values were calculated for each trait for all the genotypes. Range of mean MFV was from 0 to 1 and the higher MFV depicts higher salt tolerance.
Hierarchical cluster analysis
Furthermore, using hierarchical cluster analysis the genotypes were categorised into the following groups as per their salt tolerance levels:
Group | Salt tolerance grades/levels |
|---|---|
1 | Highly salt tolerant (HST) |
2 | Salt tolerant (ST) |
3 | Moderately salt tolerant (MST) |
4 | Salt sensitive (SS) |
5 | Highly salt sensitive (HSS) |
The mathematical evaluation model of salt tolerance was developed using SPSS software. This model is represented by the following equation
where \(\upmu =\text{ constant \, denoting \, the \, random \, error \, term},\text{ Y}=\text{mean \, MFV \, of \, genotype}, {\beta }_{i}=\text{unstandardized \, coefficient}\)
Statistical analysis
ANOVA was performed to test the level of significance amongst different concentrations. Correlation analysis between different traits was performed using IBM SPSS V.25. Using the mean MFV and STI of different traits a linear regression analysis was performed along with a mathematical evaluation model was developed by performing multiple regression analysis using SPSS software (SPSS, Chicago IL, United States).
Research involving plants
Experimental materials used in this study are cultivated varieties available in public domain and the experiment complies with relevant institutional, national, and international guidelines and legislation. No field trials were conducted.
Results
Establishment of appropriate concentration of salt stress
To determine the optimum salt concentration for screening the salt tolerance of B. juncea genotypes, 15 genotypes were randomly selected from the experimental material. After 7 days, data pertaining to the RL, SHL, SL, SFW, SDW, and GR of 15 B. juncea genotypes was collected, demonstrating the negative effect of salt stress on developing seedlings (Fig. 2).
The optimum concentration of salt stress can be assessed using the salt injury index (SII). SII provides an estimate of how much damage salinity stress causes to plants. It is the threshold at which half of the plants begin to show negative effects from salt stress. In this experiment, the salt concentration at which SII is 0.5 is regarded as the ideal concentration as the half of the seedlings show damage caused by salt stress, allowing the salinity tolerance to be characterised. Higher the SII, greater will be the damage caused by salinity stress.
Based on the recorded data, SII of each trait was calculated (Table S1). The SII of the morphological traits across all 15 B. juncea genotypes for each trait was subjected to a linear regression analysis (Fig. 3). For GR, 0.5 measure of SII was recorded at 260 mM/L NaCl treatment. Similarly, for RL, SHL, SL, SFW, and SDW, the SII of 0.5 was recorded at salt concentrations of 166.14, 168.2, 163.38, 192, and 266 mM/L respectively. The 0.5 average SII of all the morphological traits under study was recorded at 203 mM/L. Therefore, 225 mM/L NaCl treatment was considered as optimum salt stress concentration.
Selection of optimum salt concentration for evaluating salt tolerance ability of 59 Brassica juncea genotypes when salt injury index (SII) was reduced to 50% of the control. Linear fit between SII of each parameter against different NaCl concentrations (A) SII of germination rate vs NaCl (mM/L) (B) SII of Root length (RL) vs NaCl (mM/L) (C) SII of Shoot length (SHL) vs NaCl (mM/L) (D) SII of Seedling length (SL) vs NaCl (mM/L) (E) SII of Seedling fresh weight (SFW) vs NaCl (mM/L) (F) SII of Seedling dry weight (SDW) vs NaCl (mM/L) (F) Average SII vs NaCl (mM/L).
Analysis of correlation among physiological traits under stress
Using the determined optimum salt concentration (225 mM/L), the morphological data of the following traits namely, GR, RL, SHL, SL, SFW, and SDW of each genotype was measured (Table S2). STI value can be used for evaluating the impact of NaCl on salt tolerance parameters of B. juncea genotypes as it measures the ability of a plant to withstand and thrive in saline conditions. It is determined by contrasting the performance of plants in salinized and non-salinized (control) environments. Higher STI value indicates lesser impacts while lower value indicates more impact. STI values determined for the morphological traits under study are shown in the supplementary material (Table S3). A correlation analysis was performed to show the association between the morphological traits (Table 1). STI of all the morphological traits recorded were found to be positively correlated with each other. A high positive correlation was found between STI of RL and SHL (0.940), and STI of SHL and SL (0.935) which indicates the ability of the seedlings to tolerate salt stress by improving their morphological traits. Correlation for STI of SHL and SFW was 0.820, while the correlation between the STI of SL and SFW was 0.816. The weakest correlation coefficient (0.385) was found between STI of RL and SDW.
Hierarchical cluster analysis
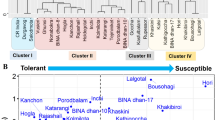
Fuzzy comprehensive evaluation method was used to obtain the MFV and mean MFV of each indicator. A higher mean MFV indicates stronger salt tolerance. A hierarchical cluster analysis based on the Furthest Neighbor was utilized to classify 59 genotypes into five categories based on their mean MFV values (Fig. 4). Out of 59 B. juncea genotypes, 4 genotypes were classified as HST, 6 genotypes as ST, 19 genotypes as MST, 21 genotypes as SS, and 9 genotypes as HSS (Fig. 5).
Classification of 59 Brassica juncea genotypes on the basis of mean membership function value (mean MFV). (A) Categorization of 59 Brassica juncea genotypes according to salt tolerance based on mean MFV. (B) Comparison between control (0 mM/L) and stress condition (225 mM/L) at various salt tolerance categories where HSS Highly salt stressed, SS Salt stressed, MST Moderately salt tolerant, ST Salt tolerant, HST Highly salt tolerant.
Establishing a mathematical model for salt tolerance evaluation
The mean MFV and STI of each morphological trait was analyzed using multiple regression analysis to establish a mathematical model for evaluating the salt tolerance. The regression equation derived is given as: Y = 0.027 + 0.207 × STIRL + 0.362 × STISHL + 0.328 × STISL + 0.345 × STISFW + 0.130 × STISDW (P < 0.01) (Table 2). In this equation values of unstandardized coefficients of STI of RL, SHL, SL, SFW and SDW are 0.207, 0.362, 0.328, 0.345 and 0.130 respectively. µ (random error term) was 0.027. Y indicates the salt tolerant ability of a certain B. juncea genotype.
Verification of the mathematical evaluation model of salt tolerance
To verify the accuracy and usefulness for predicting the salt tolerance using the established mathematical model, three genotypes were randomly selected from each salt tolerance category to calculate the Y values (Table 3). The Y values were derived for each genotype by substituting the morphological data in the mathematical model and the resultant Y values were compared with their respective mean MFV of each genotype. For example, the Y value of the #48 (SS) genotype is 0.22, and its mean MFV is also 0.22; the Y value of the #18 genotype is 0.70, and its mean MFV is also 0.71, indicating that the mean MFV and Y values are very close. Higher mean MFV value indicates high salt tolerance. The obtained results have demonstrated that the formula can be used to determine any B. juncea genotype salt tolerance at the germination stage. The B. juncea genotypes chosen to determine the ideal salt concentration can also be estimated to have salt tolerance evaluation using this model (Table S5). Therefore, STI based Y value estimation of seedling growth parameters is accurate for predicting the salt tolerance of B. juncea genotypes (Table S6).
Identification of a reliable screening trait of salt tolerance
The seedling growth parameters STIs, however, affect mean MFV indicating that mean MFV increases with an increasing STI of each seedling parameter45. To determine the most reliable morphological trait for estimating salt tolerance, a linear regression model is fitted with the STI and mean MFV of all morphological traits. Correlation coefficient (R2 = 0.859) between STI of the SFW and mean MFV (Fig. 6) was the highest (0.859) and the standardized coefficient β_SFW was also high (0.287) (Table 2) suggesting that the fresh weight of the seedling can be utilized to determine the salt tolerance of B. juncea genotypes at the seedling stage.
Linear correlation analysis between Salt tolerance index (STI) of each physiological parameter and Mean MFV. (A) Between STI of Root length and mean MFV; (B) between STI of Shoot length and mean MFV; (C) between STI of Seedling length and mean MFV; (D) between STI of Seedling fresh weight and mean MFV; (E) between STI of Seedling dry weight and mean MFV; (F) between STI of Germination rate and mean MFV. R2 Linear is the coefficient of determination.
Validation of SFW as reliable parameter
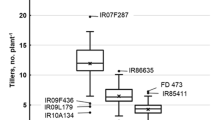
Three genotypes were selected randomly from all salt tolerance levels, and their seedling fresh weight data was recorded at optimum concentration (225 mM/L) of NaCl, to see if the parameter accurately measures salinity tolerance. There were no significant variations in growth across all genotypes in control. However, the seedling fresh weight decreased under salt stress conditions (HSS < SS < MST < ST < HST). As compared to MST and HST genotypes, which had average seedling fresh weights of 20.19 mg and 27.77 mg, respectively, HSS genotypes had an average seedling fresh weight under stress of 2.008 mg.
Discussion
Salinity stress is one of the bottlenecks in Indian mustard yield and productivity46 as it hinders plant growth at the germination stage itself resulting in lower yield and biomass47. Developing salt tolerant cultivars has proven to be a challenging and time-consuming process as stress simulation in farm is challenging and does not provide for a robust and reproducible selection criterion which significantly impacts the efficiency of the screening procedures48. Expedited screening techniques should circumvent the necessity of cultivating plants under regulated circumstances and morphological characteristics serve as the foundation for effective germplasm screening protocols. Thus, different morpho physiological parameters associated with salt tolerance can serve as criteria for evaluating and selecting plants with salt tolerance at germination and early seedling stage thus saving time and effort49,50. Various studies have explored salinity tolerance in B. napus at germination stage and report shoot fresh weight and seedling fresh weight as reliable indicators for salinity stress35,51 but no similar research has been done in Brassica juncea.
In the present study, five different salt concentrations were used to screen randomly selected genotypes of Brassica juncea at germination and seedling stage to determine a single effective salt concentration for screening purposes. It was observed that different concentrations affect fresh weight, root and shoot length in different ways. As compared to control, higher salt concentrations tend to significantly decrease the values of traits such as root, shoot and fresh weight while lower doses tend to show a slight increase42. The effects of salinity have been observed to have a significant effect on growth of root and shoot in sensitive genotypes but only to a lesser extent on tolerant ones. Sensitive genotypes shown marked reduction in dry matter accumulation due to restrained leaf area development52. The osmotic stress may be the reason of the early growth decrease in response to salt13. Significant reduction in leaf area, stem and root dry weight has been reported in a comparative study among a tolerant and sensitive Indian mustard cultivars where, salinity-tolerant cultivar showed less losses in combined dry weight and dry weights of plant components such as root and shoot40.
Utilizing either SII or STI has proven advantageous for investigating salt stress at both the phenotypic and molecular levels35,36,53 therefore on the basis of STI value we found that 225 mM/L can be used as an effective concentration to screen for salt tolerance. Subsequently, tolerance of all the genotypes was determined under 225 mM/L NaCl concentration for same parameters and analyzed using the mean membership function value (MFV). The membership function value in fuzzy set theory provides an extensive analysis by employing membership functions based on fuzzy mathematics theory and the salinity-tolerant indices (STI) of observed parameters33 and has been successfully used for salt tolerance evaluation in rapeseed34,35,51, mustard42,43, wheat54, sweet sorghum45, sunflower36, rice37 and chickpea38. All genotypes were divided into 5 categories, where in,4 genotypes were classified as HST, 6 genotypes as ST, 19 genotypes as MST, 21 genotypes as SS, and 9 genotypes as HSS (Fig. 4). The maximum mean MFV was 0.820 indicating that salinity has a bigger influence on seed germination rates across the genotypes. MFV is an important indicator for the evaluation of salt tolerance36,54, the higher the mean MFV value, the higher the salt tolerance. Some of the genotypes exhibited lower mean MFV, indicating they have high salt sensitivity at the germination stage. Plants under salt stress experience ion stress, osmotic damage, and a buildup of reactive oxygen species. It has been anticipated that the genotypes which are salt tolerant may have efficient reactive oxygen species scavenging mechanism and are able to synthesize osmoprotectants45.
A mathematical formula based upon the multiple regression analysis was used for the reliable and efficient evaluation of salt tolerance in B. juncea lines35. The mathematical model was proved to be the time saving and convenient model for screening salt tolerance. The Y value was estimated for all the lines (Table S6) and used for the accurate evaluation of salt tolerance using the model. The highest value of Y depicted the higher salt tolerance which is in accordance with the results obtained for screening of salt tolerant B. napus lines35. Correlation analysis and linear fit model were used to find the relationship between various morphological parameters under NaCl stress based on the STI values of GR, RL, SHL, SL, SFW and SDW. According to the results of the correlation analysis, the STIs of SFW under the 225 mM/L NaCl treatment presented high correlation coefficients with STI of SHL and STI of SL (0.820 and 0.816, respectively). The linear fit model showed that STI of SFW had high coefficient of determination with mean MFV (R2 = 0.859). Thus, SFW can be used as a reliable trait for screening salt-tolerant B. juncea genotypes on a large scale during the germination stage. In an assessment of Indian mustard’s early responses to salinity, kinetics of germination involving root length, shoot length and fresh weight highlighted that salinity has a strong negative correlation with fresh weight39,55. The shoot weight along with the root length and hypocotyl length were reported to be the most significant traits to find the genetic variability governing the salt tolerance among B. juncea varieties which is in accordance with this study where seedling fresh weight was found to be the most significant trait for screening of salt tolerance42.
Conclusion
To establish a reliable and accurate screening methodology for salt tolerance at the germination stage, a mathematical model was assessed and tested. Analysis of different growth parameters at germination stage across multiple salt stress concentrations using MFV revealed that seedling fresh weight (SFW) at an optimum concentration of 225 mM/L NaCl is the most reliable feature for screening salt tolerance in B. juncea. Better germination kinetics, which control biomass accumulation efficiency in developing seedlings, leads to successful earlier seedling establishment and Seedling fresh weight (SFW) might be seen as these combined processes' ultimate result. Rapid germination and strong development of seedlings increase the plant's salt resistance barrier, which is essential for sustaining plant growth and production potential. The model was used to assign a grade to each of the 59 different B. juncea, resulting in the following breakdown: 9 HSS, 4 ST, 19 MST, 21 SS, and 4 HST. Rapid, reproducible, and inexpensive identification of salt tolerant genotypes have direct implications in helping breeders in selection of parents in hybridization programs and developing salinity tolerant cultivars.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Yamaguchi, T. & Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 10, 615–620 (2005).
Shahbaz, M. & Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 32, 237–249 (2013).
Raza, A., Ashraf, F., Zou, X., Zhang, X. & Tosif, H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In Plant Ecophysiology And Adaptation Under Climate Change: Mechanisms And Perspectives Vol. 1 (ed. Hasanuzzaman, M.) (Singapore, 2020).
Ashraf, M. & McNeilly, T. Responses of four Brassica species to sodium chloride. Environ. Exp. Bot. 30, 475–487 (1990).
Chinnusamy, V., Jagendorf, A. & Zhu, J. K. Understanding and improving salt tolerance in plants. Crop Sci. 45, 437–448 (2005).
Ganie, S. A. et al. Assessment of genetic diversity of Saltol QTL among the rice (Oryza sativa L.) genotypes. Physiol. Mol. Biol. Plants. 22, 107–114 (2016).
Jamil, A., Riaz, S., Ashraf, M. & Foolad, M. R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 30, 435–458 (2011).
Shrivastava, P. & Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22, 123–131 (2015).
Mishra, S. N. & Anju, C. Nitrate and ammonium effect on Indian mustard seedling grown under salinity stress. Indian J. Plant Physiol. 1, 93–97 (1996).
Zeng, L., Shannon, M. C. & Grieve, C. M. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica. 127, 235–245 (2002).
Misra, N. & Dwivedi, U. N. Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci. 166, 1135–1142 (2004).
Flowers, T. J. Improving crop salt tolerance. J. Exp. Bot. 55, 307–319 (2004).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–668 (2008).
Hauser, F. & Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 33, 552–565 (2010).
Hanin, M., Ebel, C., Ngom, M., Laplaze, L. & Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 7, 1787 (2016).
Horie, T. et al. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 52, 663–675 (2011).
Siddiqui, M. H., Mohammad, F. & Khan, M. N. Morphological and physio-biochemical characterization of Brassica juncea L. Czern. & Coss. genotypes under salt stress. J. Plant Interact. 4, 67–80 (2009).
Epstein, E. Salt-tolerant crops: Origins, development, and prospects of the concept. Plant Soil. 89, 187–198 (1985).
Ashraf, M. Y. & Wu, L. Breeding for salinity tolerance in plants. Crit. Rev. Plant Sci. 13, 17–42 (1994).
Flowers, T. J., Garcia, A., Koyama, M. & Yeo, A. R. Breeding for salt tolerance in crop plants: The role of molecular biology. Acta Physiol. Plant. 19, 427–433 (1997).
Shannon, M. C. & Grieve, C. M. Tolerance of vegetable crops to salinity. Sci. Hortic. 78, 5–38 (1998).
FAO. & ITPS. Status of the World’s Soil Resources (SWSR)–Main Report. (2015).
Pavlović, I. et al. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage, and kale. Front. Plant Sci. 10, 450 (2019).
Kaur, P. et al. Polyphyletic origin of Brassica juncea with B. rapa and B. nigra (Brassicaceae) participating as cytoplasm donor parents in independent hybridization events. Am. J. Bot. 101, 1157–1166 (2014).
Kumar, D. The value of certain plant parameters as an index for salt tolerance in Indian mustard (Brassica juncea L.). Plant Soil. 79, 261–272 (1984).
Ashraf, M. Effect of NaCl on growth, seed protein and oil contents of some cultivars/line of brown mustard (Brassica juncea (L.) Czern and Coss). Agrochimica 36, 137–147 (1992).
Ashraf, M., Zafar, Z. U. & Tufail, M. Intra-specific variation for salt tolerance in a potential oil-seed crop, brown mustard (Brassica juncea (L.) Czern and Coss). Arch. Agron. Soil Sci. 38, 449–458 (1994).
Sharma, P., Sardana, V. & Banga, S. S. Salt tolerance of Indian mustard (Brassica juncea) at germination and early seedling growth. Environ. Exp. Biol. 11, 39–46 (2013).
Misra, A. N., Sahu, S. M. & Misra, M. Soil salinity induced changes in pigment and protein contents in cotyledons and leaves of Indian mustard [Brassica juncea Coss]. Acta Physiol. Plant. 17, 375–380 (1995).
SunitaJain, S. J., Nainawatee, H. S., Jain, R. K. & Chowdhury, J. B. Variation in ion and organic solute levels in in vitro selected NaCl-tolerant plants of Brassica juncea L. under salt stress. Indian J. Exp. Biol. 34, 554–559 (1997).
Gangopadhyay, G., Basu, S. & Gupta, S. In vitro selection and physiological characterization of NaCl-and mannitol-adapted callus lines in Brassica juncea. Plant Cell Tissue Organ Cult. 50, 161–169 (1997).
Wright, P. R., Morgan, J. M. & Jessop, R. S. Turgor maintenance by osmoregulation in Brassica napus and B. juncea under field conditions. Ann. Bot. 80, 313–319 (1997).
Zadeh, L. Fuzzy sets. Inform. Control 8, 338–353 (1965).
Long, W. H., Pu, H. M., Zhang, J. F., Qi, C. K. & Zhang, X. K. Screening of Brassica napus for salinity tolerance at germination stage. Chin. J. Oil Crop Sci. 35, 271–275 (2013).
Wu, H. et al. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 10, 530 (2019).
Li, W. et al. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 10, 10626 (2020).
Zhang, R. et al. Comprehensive evaluation of salt tolerance in rice (Oryza sativa L.) germplasm at the germination stage. Agronomy 11, 1569 (2021).
Kaur, H., Kaur, R., Manchanda, G., Bindra, S. & Sharma, A. Screening of Cicer arietinum L. genotypes under combined presence of NaCl and anthracene using membership function value of stress tolerance. Plant Gene. 31, 100371 (2022).
Pandey, M. & Penna, S. Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassica juncea L. Crop J. 5, 219–230 (2017).
Singh, B. K. et al. Comparative analysis for understanding salinity tolerance mechanism in Indian mustard (Brassica juncea L.). Acta Physiol. Plant. 41, 1–14 (2019).
Bandopadhyay, L., Mukherjee, S. & Sikdar, S. R. Estimating the salt tolerance level of Brassica juncea B-85 (Seeta) variety by physiological and biochemical analysis. Plant Physiol. Rep. 26, 374–380 (2021).
Li, H., Feng, H., He, Y., Liu, J. & Zhu, Y. Screening of leaf mustard (Brassica juncea L.) cultivars for salinity tolerance. Commun. Soil Sci. Plant Anal. 54, 2657–2674 (2023).
Kumawat, G. et al. High throughput phenotyping of functional traits and key indices for selection of salt tolerant Mustard [Brassica juncea (L.) Czern & Coss] genotypes. Physiol. Plant. 176, e14178 (2024).
Chen, X. J., Min, D. H., Yasir, T. A. & Hu, Y. G. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 137, 195–201 (2012).
Ding, T. L. et al. Evaluation of salt-tolerant germplasm and screening of the salt-tolerance traits of sweet sorghum in the germination stage. Funct. Plant Biol. 45, 1073–1081 (2018).
Singh, J. et al. Differential response of Indian mustard (Brassica juncea L., Czern and Coss) under salinity: Photosynthetic traits and gene expression. Physiol. Mol. Biol. Plants 25, 71–83 (2019).
Hamdy, A., Abdel-Dayem, S. & Abu-Zeid, M. Saline water management for optimum crop production. Agric. Water Manag. 24, 189–203 (1993).
Khanna, A. et al. Genetic gains in IRRI’s rice salinity breeding and elite panel development as a future breeding resource. Theor. Appl. Genet. 137, 1–14 (2024).
Guo, J., Du, M., Tian, H. & Wang, B. Exposure to high salinity during seed development markedly enhances seedling emergence and fitness of the progeny of the extreme halophyte Suaeda salsa. Front. Plant Sci. 11, 1291 (2020).
Tarchoun, N. et al. The effects of salt stress on germination, seedling growth and biochemical responses of tunisian squash (Cucurbita maxima Duchesne) germplasm. Plants. 11, 800 (2022).
Bakirov, A. et al. Screening of salt tolerance traits and the salt tolerance evaluation method in Brassica napus at the seed germination stage. Ital. J. Agron. 17, 2011 (2022).
Javid, M., Ford, R. & Nicolas, M. E. Tolerance responses of Brassica juncea to salinity, alkalinity and alkaline salinity. Funct. Plant Biol. 39, 699–707 (2012).
Wassan, G. M. et al. Identification of genetic variation for salt tolerance in Brassica napus using genome-wide association mapping. Mol. Genet. Genom. 296, 391–408 (2021).
Choudhary, A., Kaur, N., Sharma, A. & Kumar, A. Evaluation and screening of elite wheat germplasm for salinity stress at the seedling phase. Physiol. Plant 173, 2207–2215 (2021).
Han, X. et al. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 64, 4589–4601 (2013).
Author information
Authors and Affiliations
Contributions
P.W. and S.S.S. designed the experiment, G.A. and P.E. executed the experiments; P.E. and S.J. analysed the data. G.A. and P.E. wrote first draft of manuscript. P.W., S.S.S. and S.J. revised and finalised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aggarwal, G., Edhigalla, P., Walia, P. et al. A method for screening salt stress tolerance in Indian mustard (Brassica juncea) (L.) Czern & Coss at seedling stage. Sci Rep 14, 12705 (2024). https://doi.org/10.1038/s41598-024-63693-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63693-6
- Springer Nature Limited