Abstract
The TNO Gastro-Intestinal Model (TIM) is a multi-compartmental model, designed to realistically simulate conditions in the lumen of the gastro-intestinal tract. TIM is successfully used to study the gastro-intestinal behavior of a wide variety of feed, food and pharmaceutical products. Experiments in TIM are based on a computer simulation of the digestive conditions in the lumen of the gut during transit and digestion of a meal in vivo. These conditions include controlled parameters such as gastric and small intestinal transit, flow rates and composition of digestive fluids, pH values, and removal of water and metabolites. Simulation protocols have been developed for young, adult and elderly humans, dogs, pigs and calves after ingestion of various meals. The typical end point from results obtained with TIM is the availability of a compound for absorption through the gut wall (bio-accessibility). Results from TIM—with or without additional intestinal cell assays and in silico modeling—show a high predictability as compared to in vivo data (Marteau et al., J Dairy Sci 80:1031–1037, 1997; Verwei et al., J Nutr 136:3074–2078, 2006; Bellmann et al., TIM-carbo: a rapid, cost-efficient and reliable in vitro method for glycaemic response after carbohydrate ingestion. In: van der Kamp J-W, Jones JM, McCleary BV (eds) Dietary fibre: new frontiers for food and health. Wageningen Academic Publishers, Wageningen, p 467–473, 2010; Van Loo-Bouwman et al., J Agric Food Chem 62(4):950–955, 2014).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Multi-compartmental dynamic gastric intestinal model
- Physiological
- Gastric
- In vitro
- Digestion
- Bio-accessibility
- Nutrient
- Digestion
1 Introduction
The TNO Gastro-Intestinal Model (TIM) is a multi-compartmental dynamic model that was developed in the early 1990s in response to industrial demand to study food products under more physiologically relevant conditions as compared to contemporary digestion models (Minekus et al. 1995). During the past years TIM has developed from an experimental lab setup—controlled by an 8 MHz PC—into a platform of cabinet systems that are successfully used for a broad range of studies, serving the feed, food and pharmaceutical industries. This chapter describes the concept of TIM, the TIM gastro-intestinal systems and some examples of methods to study the digestion of nutrients.
2 Concept of TIM
The gastro-intestinal tract is a tube like organ with different compartments (stomach, small intestine, large intestine) for each step of digestion. During the gradual transit of the meal through the compartments different fractions of the meal are exposed to changing conditions due to gradual secretion of digestive fluids and absorption of water and nutrients. TIM intends to simulate the dynamic conditions in the lumen of the gastrointestinal tract. It is designed to combine the controllability and reproducibility of a model system with physiological parameters such as mixing, meal transit, variable pH values in place and time, realistic secretion and composition of digestive fluids, and removal of digested compounds and water. These parameters are combined in a protocol as an input for a computer simulation of a specific digestive setting. Such settings includes species (human, dog, pig, calve), age (infant, adult, elderly), pathology and meal-related parameters, obtained from in vivo data (Marteau et al. 1997; Minekus 1998; Smeets-Peeters 2000; Havenaar et al. 2013). Based on the computer simulation, the physical model is controlled to reproduce the underlying in vivo settings.
3 TIM-1
TIM-1 (Fig. 5.1) is the most frequently used configuration of the TIM platform. It comprises four compartments, representing the stomach, duodenum, jejunum and ileum. Compartments are connected by peristaltic valve pumps (PVP) that allow the transfer of controlled amounts of chyme. The PVPs are designed to have low dead volume in the closed position. They are not blocked by particles and able to handle complete meals. Mixing for each compartment is achieved by alternating the pressure on flexible walls. Temperature is maintained by controlling the temperature of the water circulating outside the flexible walls. Prior to introduction into the gastric compartment, the meal is masticated with a food processor (Solostar II, Tribest) and mixed with artificial saliva containing electrolytes and α-amylase. Gastric secretion contains electrolytes, pepsin and a fungal lipase (F-AP 15, Amano) as an alternative to gastric lipase. The pH is measured and controlled with hydrochloric acid, to follow a predetermined curve or at a variable rate in time. Duodenal secretion consists of, electrolytes, bile and pancreatin. The pH is controlled at pre-set values for each compartment with sodium bicarbonate. All flows of secretion are programmable in time. Digestion products are removed by two different systems. Water soluble products are removed by dialysis through membranes with a molecular weight cutoff of app. 10 kDa, connected to the jejunal and ileal compartments. Lipophilic products cannot be removed efficiently by these membranes since they are incorporated in micelles that are too big to pass the membrane. Lipophilic products are removed through a 50 nm filter that passes micelles but retains fat droplets. Meal transit is controlled by dictating the gastric- and ileal-emptying according to the formula (Fig. 5.2) described by Elashoff et al. (1982). A typical protocol for the simulation of the digestion of a high fat meal in a human adult is presented in Table 5.1. An overview of the composition of physiological relevant secreted fluids for a human adult is given in Chap. 2 on the Infogest consensus method for static digestion.
Schematic presentation of TIM-1, equipped with filters to study the bio-accessibility of lipids. A. gastric compartment; B. pyloric sphincter; C. duodenal compartment; D. peristaltic valve; E. jejunal compartment; F. peristaltic valve; G. ileal compartment; H. ileal-cecal valve; I. gastric secretion; J. duodenal secretion; K. bicarbonate secretion; L. pre-filter; M. filtration system; N. filtrate with bio-accessible fraction; O. hollow fiber system (cross section); P. pH electrodes; Q. level sensors; R. temperature sensors; S. pressure sensor
4 TinyTIM
TinyTIM (Fig. 5.3) is a simplified version of the TIM-1, designed to increase the throughput as compared to TIM-1, with focus on studies that do not need separate intestinal steps. The TinyTIM is used with the same gastric compartment as TIM-1 when the ratio between amount of food and ingested material, such as pharmaceutical formulations, is important. For other experiments, a half size gastric compartment is used. All functions of the gastric compartment are similar to the gastric compartment of TIM-1. TinyTIM has a single small intestinal compartment instead of three and no ileal efflux. All fluids entering the small intestinal compartment are removed through the filtration- or dialysis-membrane. This implies that small intestinal transit is simulated by assuming a plug of chyme traveling through the small intestine, instead of a “flow through” compartment such as in TIM-1.
Schematic presentation of TinyTIM, equipped with a dialysis membrane to study the bio-accessibility of water soluble compounds. A. gastric compartment; B. pyloric sphincter; C. duodenal compartment; D. gastric secretion; E. duodenal secretion; F. pre-filter; G. pH electrodes; H. dialysis membrane; I. dialysis system; J. pressure sensor; K. level sensor
5 Advanced Gastric Compartment (TIM-agc)
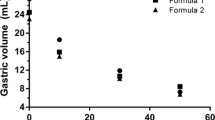
In the standard gastric compartment, the meal is mixed to obtain a homogenized gastric content and a consequent predictable gastric emptying of compounds. This is particularly important to compare the digestion of compounds under exactly controlled conditions. In order to include the effect of gastric motility on the gastric behavior of food components and pharmaceuticals, a gastric compartment is designed that mimics the shape and motility of the stomach in a more realistic manner (Fig. 5.4). The system consists of a body part with a flexible wall that gradually contracts to simulate gastric tone and consequent reduction of gastric volume during emptying. Two antral units can be moved to simulate mixing by an antral wave. A valve is synchronized with an antral wave to simulate the opening of the pyloric sphincter during gastric emptying. Similar to other TIM models, the contractions are achieved by modulating the pressure on water that is circulated in the space between a glass jacket and a flexible membrane. All contractile movements and the resulting mixing and pressure profiles are accurately controlled and synchronized. Motility patterns as well as gastric emptying and secretion of digestive fluids are dictated by a predetermined protocol that describes a specific condition (e.g. fed or fasting) in time. A study has been performed in both the TIM-agc and human volunteers to compare gastric pressure profiles, using a smartpill® (Given Imaging GmbH, Hamburg, Germany) (Fig. 5.5, Minekus et al. 2013).
6 The Use of TIM to Study the Bio-accessibility of Nutrients
The TIM has been successfully used to study the bio-accessibility of macro nutrients, minerals, fat- and water soluble vitamins, and bioactive compounds (Larsson et al. 1999; Verwei et al. 2006; Mateo Anson et al. 2009). Bio-accessibility is defined as the fraction of a compound that is available for absorption through the gut wall. In TIM, this is determined by measuring the fraction of a compound that has passed the dialysis or filtration membrane. When validating TIM with in vivo data, it is important to realize that a valid correlation between bio-accessibility and bio-availability can only be achieved when transport through the gut wall (mucus layer and enterocytes) is not a limiting step. This gap can be bridged by using TIM data in combination with transport data obtained with intestinal cells and/or in silico modeling. The digestion of a nutrient is determined by the characteristics of the nutrient, the composition and structure of the meal matrix and the individual’s physical response to the digesting nutrient and meal while travelling through the gastro-intestinal tract. As is the case in all digestive models, TIM does not include feedback on the characteristics of the meal. Therefore, the approach is taken to assume a set of conditions that is based on in vivo data and normal for the type of meal and target group. The effect of variability of a specific condition on digestion within a population can be tested by changing only this condition in the digestive protocol. The reproducible conditions allow comparison of different compounds under the same conditions and do not need as many replicates as are necessary to obtain sufficient statistical power for in vivo studies.
7 Protein Quality
The basic method to study protein digestion in TIM is to determine the bio-accessibility by expressing the amount of protein nitrogen dialyzed as a percentage of the amount of protein nitrogen in the meal. The bio-accessibility data are corrected with the bio-accessibility of protein nitrogen from the secreted protein, thus presenting the true digestibility of the protein.
To optimize the nutritional quality of food and feed, it is important for the food and feed industries to have insight in the nutritional quality of the protein in their ingredients. The nutritional quality of proteins depends on the amino acid composition profile and the bio-accessibility of essential amino acids, while digestion of proteins may be affected by processing steps during manufacturing. Essential amino acids cannot be produced by the body and need to be supplied in sufficient quantities in the diet. A protein of high biological value contains all essential amino acid in proportion to the need. The amino acid that is in shortest supply in relation to the need is referred to as the limiting amino acid. Traditionally protein quality is evaluated by determination of the Protein Efficiency Ratio (PER). The PER method reflects the amino acid requirements of young animals such as broiler chickens and rats, as determined with growth experiments on protein sources (Skinner et al. 1991). However, these experiments are relatively slow and do not give insight into the availability of the relevant amino acids. Also, this method determines the requirements of rats and broilers, not humans. Moreover, such experiments can result in strong growth retardation due to amino acid deficiencies and have therefore ethical drawbacks. A method was developed with TinyTIM as an alternative to the time consuming PER test that uses young animals. In this method the bio-accessible amount of the limiting amount of amino acid is determined after digesting the feed in TinyTIM (Minekus and Van der Klis 2001; Minekus et al. 2006). The FAO/WHO adopted the Protein Digestibility Corrected Amino Acid Score (PDCAAS) and later the Digestible Indispensable Amino Acid Score (DIAAS) as best method to determine the protein quality (Schaafsma 2005; FAO 2013). The method with TinyTIM to determine the true digestibility of protein and (limiting) amino acids offers an alternative to the use of rats for the determination of the PDCAAS and DIAAS, respectively.
8 Prediction of Glycemic Response
Studies on the digestion of carbohydrates and consequent glucose plasma levels are important for diabetic patients, obesity control and designing sport foods. As an alternative to expensive and time consuming human trials, a rapid in vitro method has been developed to predict the glycemic response after intake of carbohydrates (Bellmann et al. 2010). In this method the carbohydrates are digested in TinyTIM and a successive step with brush border enzymes. The released glucose and fructose are analyzed as a function of time and processed with in silico modeling based on the homeostatic model assessment (HOMA; Matthews et al. 1985) to predict the glycemic response. The method was validated against in vivo plasma glucose curves of 21 different food products (R = 0.91).
9 Lipids
The uptake of fat soluble compounds needs a realistic simulation of the digestion of the compound and the food matrix, and the formation of mixed micelles. In TIM, fresh porcine bile is used to supply adequate quantities of bile salts, phospholipids and cholesterol for mixed micelle formation. Filters with a pore size of 50 nm are used to differentiate between undigested fat and micelles, and to remove lipolytic products to avoid product inhibition. It is assumed that the products in micelles are available for absorption through the gut wall. Figure 5.6 shows the cumulative appearance of total fatty acids in the jejunal and ileal filtrates during digestion of intra lipid in TIM-1. This method has been used to study the bio-accessibility of carotenoids (Southon 2001; Van Loo-Bouwman et al. 2014), the study of Partially Hydrolysed Guar Gum (PHGG) on lipid digestion (Minekus et al. 2005) and the bio-accessibility of blueberry anthocyanins (Ribnicky et al. 2014).
10 Conclusions
TIM is designed to reproduce the conditions in the lumen of the gastro-intestinal tract by realistic mixing, transit of the meal, rate and composition of secretions and removal of digested products and water. It is designed to predict the bio-accessibility of a wide variety of ingested compounds present in a broad range of foods and pharmaceutical matrices. Accurate simulation and control of the multi-compartmental processes in the lumen of the GIT allows the testing of compounds under exactly the same conditions or specifically modified conditions for “what if” studies. Bio-accessibility profiles directly from TIM or after a separate transport assay with intestinal cells can be processed further with in silico modeling to predict the bio-availability of compounds. In contrast with static methods, that are only useful for specific studies within a narrow range of products (Minekus et al. 2014), TIM is intended to simulate the dynamic conditions in the lumen of the GIT to predict the bio-accessibility of a variety of nutrients in a wide range of meals. The more complex system with a lower throughput and higher costs is well compensated by the high predictability and broad applicability. As a high-end digestion model system, it may offer a faster and a more ethical alternative to studies in animals and humans.
Contract research on gastro-intestinal behavior of nutrients and pharmaceuticals, as well as sales and lease of TIM equipment is located at TNO-Triskelion, Zeist, The Netherlands (http://www.tnotriskelion.com/).
References
Bellmann S, Minekus M, Zeijdner E, Verwei M, Sanders P, Basten W, Havenaar R (2010) TIM-carbo: a rapid, cost-efficient and reliable in vitro method for glycaemic response after carbohydrate ingestion. In: van der Kamp J-W, Jones JM, McCleary BV (eds) Dietary fibre: new frontiers for food and health. Wageningen Academic Publishers, Wageningen, pp 467–473
Elashoff JD, Reedy TJ, Meyer JH (1982) Analysis of gastric emptying data. Gastroenterology 83(6):1306–1312
FAO Food and Nutrition Paper 92. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation’, Rome 2013
Havenaar R, Anneveld B, Hanff LM, de Wildt SN, de Koning BAE, Mooij MG, Lelieveld JPA, Minekus M (2013) In vitro gastrointestinal model (TIM) with predictive power, even for infants and children? Int J Pharm http://dx.doi.org/10.1016/j.ijpharm.2013.07.053
Larsson M, Minekus M, Havenaar R (1999) Estimation of the bioavailability of iron and phosphorus in cereals using a dynamic in vitro gastrointestinal model. J Sci Food Agric 74(1):99–106
Marteau P, Minekus M, Havenaar R, Huis in’t Veld JHJ (1997) Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci 80:1031–1037
Mateo Anson N, Havenaar R, Bast A, Haenen GRMM (2009) Antioxidant and anti-inflammatory capacity of bioaccessible compounds from wheat fractions after gastrointestinal digestion. J Cereal Sci 51(1):110–114
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Minekus M (1998) Development and validation of a dynamic model of the gastrointestinal tract. PhD thesis, University of Utrecht, Elinkwijk b.v., Utrecht, Netherlands
Minekus M, van der Klis JD (2001) Development and validation of a dynamic in vitro method as alternative to PER studies in chickens. TNO report V4326
Minekus M, Marteau P, Havenaar R, Huis in’t Veld J (1995) A multi-compartmental dynamic computer-controlled model simulating the stomach and small intestine. ATLA 23:197–209
Minekus M, Jelier M, Xiao JZ, Kondo S, Iwatsuki K, Kokubo S, Bos M, Dunnewind B, Havenaar R (2005) Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Biosci Biotechnol Biochem 69(5):932–938
Minekus M, Lelieveld J, Havenaar R (2006) Rapid, reliable and cost-effective methodology for measuring feed quality. In: Proceeding VIV Europe, Utrecht, Netherlands
Minekus M, Lelieveld J, Anneveld B, Zeijdner E, Barker R, Banks S, Miriam Verwei M (2013) Pressure forces in the TIM-agc, an advanced gastric compartment that simulates shape and motility of the stomach. Poster 5th WCDATD/OrBiTo Meeting Uppsala, Sweden, 24–27 June
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carriere F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, Macierzanka A, Mackie A, Marze S, McClements DJ, Menard O, Recio I, Santos CN, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Brodkorb A (2014) A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. http://dx.doi.org/10.1039/C3FO60702J
Ribnicky DM, Roopchand DE, Oren A, Grace M, Poulev A, Lila MA, Havenaar R, Raskin I (2014) Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem 142:349–357
Schaafsma G (2005) The protein digestibility-corrected amino acid score (PDCAAS) – a concept for describing protein quality in foods and food ingredients: a critical review. J AOAC Int 88(3):988–994
Skinner JT, Izat AL, Waldroup PW (1991) Effects of dietary amino acid levels on performance and carcass composition of broilers 42 to 49 days of age. Poult Sci 70(5):1223–1230
Smeets-Peeters MJE (2000) Feeding FIDO: development, validation and application of a dynamic in vitro model of the gastrointestinal tract of the dog. PhD thesis, Wageningen University, Universal Press, Veenendaal, The Netherlands
Southon S (2001) Model systems in vitro and in vivo, for predicting the bioavailability of lipid soluble components in food. FAIR CT97-3100 (Final Report)
Van Loo-Bouwman CA, Naber THJ, Minekus M, van Breemen RB, Hulshof PJM, Schaafsma G (2014) Matrix effects on bioaccessibility of β-carotene can be measured in an in vitro gastrointestinal model. J Agric Food Chem 62(4):950–955
Verwei M, Freidig AP, Havenaar R, Groten JP (2006) Predicted plasma folate levels based on in vitro studies and kinetic modeling consistent with measured plasma profiles in humans. J Nutr 136:3074–3078
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Minekus, M. (2015). The TNO Gastro-Intestinal Model (TIM). In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)