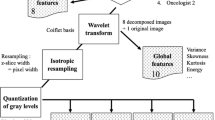
Abstract
The aim of this study was to investigate the robustness of radiomics features extracted from computed tomography (CT) images of patients affected by non-small-cell lung carcinoma (NSCLC). Specifically, the impact of manual segmentation on radiomics feature values and their variability were assessed. Therefore, 63 patients affected by squamous cell carcinoma (SCC) and adenocarcinoma (ADC) were retrospectively collected from a public dataset. Original segmentations (automated plus manual refinement approach) were provided together with CT images. Through the matRadiomics tool, manual segmentation of the volume of interest (VOI) was repeated by two training physicians and 107 features were extracted. Feature extraction was also performed using the original segmentations. Therefore, three datasets of extracted features were obtained and compared computing the difference percentage coefficient (DP) and the intraclass correlation coefficient (ICC). Moreover, feature reduction and selection on each dataset were performed using a hybrid descriptive inferential method and the differences among the three feature subsets were evaluated. Successively, three classification models were obtained using the Linear Discriminant Analysis (LDA) classifier. Validation was performed through 10 times repeated 5-fold stratified cross validation. As result, even if 87% features obtained an ICC > 0.8, showing robustness, an AVGDP (averaged DP) equal to 16.2% was observed between the datasets based on manual segmentation. Moreover, manual segmentation had an impact on the subsets of selected features, thus influencing study reproducibility and model explainability.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Siegel, R.L., Miller, K.D., Fuchs, H.E., Jemal, A.: Cancer statistics, 2022. Cancer J. Clin. 72, 7–33 (2022). https://doi.org/10.3322/caac.21708
Dalmartello, M., et al.: European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 33, 330–339 (2022). https://doi.org/10.1016/j.annonc.2021.12.007
Siegel, R.L., Miller, K.D., Wagle, N.S., Jemal, A.: Cancer statistics, 2023. Cancer J. Clin. 73, 17–48 (2023). https://doi.org/10.3322/caac.21763
Duma, N., Santana-Davila, R., Molina, J.R.: Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 94, 1623–1640 (2019). https://doi.org/10.1016/j.mayocp.2019.01.013
Travis, W.D., et al.: The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thoracic Oncol. 10, 1243–1260 (2015). https://doi.org/10.1097/JTO.0000000000000630
Xing, P.-Y., et al.: What are the clinical symptoms and physical signs for non-small cell lung cancer before diagnosis is made? A nation-wide multicenter 10-year retrospective study in China. Cancer Med. 8, 4055–4069 (2019). https://doi.org/10.1002/cam4.2256
Vernuccio, F., Cannella, R., Comelli, A., Salvaggio, G., Lagalla, R., Midiri, M.: [Radiomics and artificial intelligence: new frontiers in medicine.]. Recenti Prog. Med. 111, 130–135 (2020). https://doi.org/10.1701/3315.32853
Mayerhoefer, M.E., et al.: Introduction to radiomics. J. Nucl. Med. 61, 488–495 (2020). https://doi.org/10.2967/jnumed.118.222893
Cuocolo, R., et al.: Machine learning applications in prostate cancer magnetic resonance imaging. Eur. Radiol. Exp. 3, 35 (2019). https://doi.org/10.1186/s41747-019-0109-2
Comelli, A., et al.: Radiomics: a new biomedical workflow to create a predictive model. In: Papież, B.W., Namburete, A.I.L., Yaqub, M., Noble, J.A. (eds.) Medical Image Understanding and Analysis. Communications in Computer and Information Science, vol. 1248, pp. 280–293. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-52791-4_22
Alongi, P., et al.: 18F-Florbetaben PET/CT to assess Alzheimer’s disease: a new analysis method for regional amyloid quantification. J. Neuroimaging 29, 383–393 (2019). https://doi.org/10.1111/jon.12601
Shu, Z.-Y., et al.: Predicting the progression of Parkinson’s disease using conventional MRI and machine learning: an application of Radiomic biomarkers in whole-brain white matter. Magn. Reson. Med. 85, 1611–1624 (2021). https://doi.org/10.1002/mrm.28522
Nepi, V., Pasini, G., Bini, F., Marinozzi, F., Russo, G., Stefano, A.: MRI-Based Radiomics analysis for identification of features correlated with the expanded disability status scale of multiple sclerosis patients. In: Mazzeo, P.L., Frontoni, E., Sclaroff, S., and Distante, C. (eds.) Image Analysis and Processing. ICIAP 2022 Workshops, pp. 362–373. Springer International Publishing, Cham (2022). https://doi.org/10.1007/978-3-031-13321-3_32
Zwanenburg, A., et al.: The image biomarker standardization initiative: standardized quantitative Radiomics for high-throughput image-based phenotyping. Radiology. 295, 328–338 (2020). https://doi.org/10.1148/radiol.2020191145
Stefano, A., et al.: Robustness of PET Radiomics features: impact of co-registration with MRI. Appl. Sci. 11, 10170 (2021). https://doi.org/10.3390/app112110170
van Timmeren, J.E., Cester, D., Tanadini-Lang, S., Alkadhi, H., Baessler, B.: Radiomics in medical imaging—”how-to” guide and critical reflection. Insights Imag. 11, 91 (2020). https://doi.org/10.1186/s13244-020-00887-2
Cutaia, G., et al.: Radiomics and prostate MRI: current role and future applications. J. Imag. 7, 34 (2021). https://doi.org/10.3390/jimaging7020034
Pasini, G., Stefano, A., Russo, G., Comelli, A., Marinozzi, F., Bini, F.: Phenotyping the histopathological subtypes of non-small-cell lung carcinoma: how beneficial is Radiomics? Diagnostics. 13, 1167 (2023). https://doi.org/10.3390/diagnostics13061167
Primakov, S.P., et al.: Automated detection and segmentation of non-small cell lung cancer computed tomography images. Nat. Commun. 13, 3423 (2022). https://doi.org/10.1038/s41467-022-30841-3
Stefano, A., et al.: A preliminary PET radiomics study of brain metastases using a fully automatic segmentation method. BMC Bioinform. 21, 325 (2020). https://doi.org/10.1186/s12859-020-03647-7
Comelli, A., et al.: Development of a new fully three-dimensional methodology for tumours delineation in functional images. Comput. Biol. Med. 120, 103701 (2020). https://doi.org/10.1016/j.compbiomed.2020.103701
Comelli, A., et al.: Tissue classification to support local active delineation of brain tumors. In: Zheng, Y., Williams, B.M., Chen, K. (eds.) Medical Image Understanding and Analysis. Communications in Computer and Information Science, vol. 1065, pp. 3–14. Springer, Cham (2020). https://doi.org/10.1007/978-3-030-39343-4_1
Banna, G.L., et al.: Predictive and prognostic value of early disease progression by PET evaluation in advanced non-small cell lung cancer. Oncology 92, 39–47 (2017). https://doi.org/10.1159/000448005
Stefano, A., et al.: A fully automatic method for biological target volume segmentation of brain metastases. Int. J. Imag. Syst. Technol. 26, 29–37 (2016). https://doi.org/10.1002/ima.22154
Stefano, A., et al.: A graph-based method for PET image segmentation in radiotherapy planning: a pilot study. In: Petrosino, A. (ed.) Image Analysis and Processing – ICIAP 2013. Lecture Notes in Computer Science, vol. 8157, pp. 711–720. Springer, Heidelberg (2013). https://doi.org/10.1007/978-3-642-41184-7_72
Agnello, L., Comelli, A., Ardizzone, E., Vitabile, S.: Unsupervised tissue classification of brain MR images for voxel-based morphometry analysis. Int. J. Imaging Syst. Technol. 26, 136–150 (2016). https://doi.org/10.1002/ima.22168
Bakr, S., et al.: Data for NSCLC Radiogenomics collection (2017). https://wiki.cancerimagingarchive.net/x/W4G1AQ, https://doi.org/10.7937/K9/TCIA.2017.7HS46ERV
Pasini, G., Bini, F., Russo, G., Comelli, A., Marinozzi, F., Stefano, A.: MatRadiomics: a novel and complete Radiomics framework, from image visualization to predictive model. J. Imag. 8, 221 (2022). https://doi.org/10.3390/jimaging8080221
Bakr, S., et al.: A Radiogenomic dataset of non-small cell lung cancer. Sci Data. 5, 180202 (2018). https://doi.org/10.1038/sdata.2018.202
van Griethuysen, J.J.M., et al.: Computational Radiomics system to decode the radiographic phenotype. Can. Res. 77, e104–e107 (2017). https://doi.org/10.1158/0008-5472.CAN-17-0339
Haralick, R.M., Shanmugam, K., Dinstein, I.: Textural features for image classification. IEEE Trans. Syst., Man Cybern. SMC-3, 610–621 (1973). https://doi.org/10.1109/TSMC.1973.4309314
Galloway, M.M.: Texture analysis using gray level run lengths. Comput. Graph. Image Process. 4, 172–179 (1975). https://doi.org/10.1016/S0146-664X(75)80008-6
Thibault, G., Angulo, J., Meyer, F.: Advanced statistical matrices for texture characterization: application to cell classification. IEEE Trans. Biomed. Eng. 61, 630–637 (2014). https://doi.org/10.1109/TBME.2013.2284600
Amadasun, M., King, R.: Textural features corresponding to textural properties. IEEE Trans. Syst. Man Cybern. 19, 1264–1274 (1989). https://doi.org/10.1109/21.44046
Sun, C., Wee, W.G.: Neighboring gray level dependence matrix for texture classification. Comput. Vis., Graph. Image Process. 23, 341–352 (1983). https://doi.org/10.1016/0734-189X(83)90032-4
McGraw, K.O., Wong, S.P.: Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1, 30–46 (1996). https://doi.org/10.1037/1082-989X.1.1.30
Barone, S., et al.: Hybrid descriptive-inferential method for key feature selection in prostate cancer Radiomics. Appl. Stoch. Model. Bus. Ind. 37, 961–972 (2021). https://doi.org/10.1002/asmb.2642
Comelli, A., et al.: Active contour algorithm with discriminant analysis for delineating Tumors in positron emission tomography. Artif. Intell. Med. 94, 67–78 (2019). https://doi.org/10.1016/j.artmed.2019.01.002
Parmar, C., et al.: Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS ONE 9, e102107 (2014). https://doi.org/10.1371/journal.pone.0102107
Acknowledgements
The author thanks the two training physicians, namely Accursio Scaduto and Francesco Cutrì, for having inspected the DICOM volume, manually segmented the NSCLCs and for feature extraction.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Pasini, G. (2024). Assessing the Robustness and Reproducibility of CT Radiomics Features in Non-small-cell Lung Carcinoma. In: Foresti, G.L., Fusiello, A., Hancock, E. (eds) Image Analysis and Processing - ICIAP 2023 Workshops. ICIAP 2023. Lecture Notes in Computer Science, vol 14366. Springer, Cham. https://doi.org/10.1007/978-3-031-51026-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-51026-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-51025-0
Online ISBN: 978-3-031-51026-7
eBook Packages: Computer ScienceComputer Science (R0)