Abstract
Intravenous fluids are one of the most important parts of the multimodal strategy used in resuscitation that helps to improve tissue perfusion. There is a long controversy regarding the usage of crystalloids. It is unlikely that one balanced solution is better than another and the choice of fluid should be based on clinician preference.
“Each time new experiments are observed to agree with the predictions, the theory survives and our confidence in it is increased; but if ever new observation is found to disagree, we have to abandon or modify the theory”
—Stephen Hawking,
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara IFA Commentary (MLNGM)Buffered solutions are commonly used in medical settings to help maintain acid–base balance in patients. The choice of buffer can impact the effectiveness and safety of the solution. The ideal solution to maintain pH should have a strong ion difference (SID) of around 24–28 mmol/L, whereas abnormal saline has a SID of zero and hereby can induce hyperchloremic metabolic acidosis. Lactate, malate, acetate, gluconate and pyruvate are all potential buffer choices for balanced solutions (Figure). The selection of a particular buffer is influenced by several factors, including pH, the desired buffering capacity and the safety profile of the buffer. Lactate is a commonly used buffer in balanced solutions. It has a pKa of 3.9, which allows it to buffer acidic conditions effectively. Lactate is also metabolized to bicarbonate in the liver, providing an additional mechanism for regulating acid–base balance. Malate is another buffer option with a pKa of 3.2. It can buffer both acidic and basic conditions effectively and is metabolized in the liver to bicarbonate. However, malate can also increase aluminum absorption, which may be a concern for some patients. Acetate has a pKa of 4.8 and can buffer acidic conditions effectively. It is also metabolized in the liver to bicarbonate. Acetate is generally considered safe, but large doses may cause metabolic acidosis. Gluconate has a pKa of 4.4 and can buffer both acidic and basic conditions. It is metabolized to CO2 and water and is generally considered safe. However, gluconate can increase potassium levels, which may be a concern in patients with renal impairment. Pyruvate has a pKa of 2.5 and can buffer acidic conditions effectively. It is metabolized to bicarbonate in the liver and has been investigated for use in resuscitation fluids. However, high doses of pyruvate have been associated with hemodynamic instability and metabolic acidosis. Overall, the choice of buffer in balanced solutions should be based on the individual patient’s needs and safety profile.

After reading this chapter, you will:
-
1.
Understand the importance of buffers in balanced solutions.
-
2.
Understand the role of different types of buffers in maintaining acid–base balance.
-
3.
Understand the potential benefits and drawbacks of different buffers in clinical settings.
Mr. X, aged 47 years, is admitted from the ED for the management of sepsis. He was resuscitated with 30 mL/kg of 0.9% normal saline. He was started on norepinephrine infusion as he was poorly responsive to fluids. Estimation of his right atrial pressure via inferior vena cava triggers many more liters of intravenous saline. His morning blood analysis results: Cl-120 mEq/L, HCO3–12 mEq/L, lactate-5.5 mEq/L. He was anuric, hypoxemic, acidemic and on high-flow oxygen via nasal cannulae.
What are the possible reasons for his metabolic acidosis? Are balanced solutions better than normal saline? Which balanced solution is preferable amongst those available?
Introduction
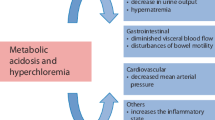
The 2021 Surviving Sepsis Guidelines suggested an immediate resuscitation in a shocked patient, to maintain perfusion of tissues. Intravenous fluids are integral to the multimodal strategy used in resuscitation. Despite their widespread usage, there remains uncertainty about the relative safety of various intravenous fluids. “Normal” saline, i.e. 0.9% sodium chloride (NaCl) was developed by Dutch physiologist Dr. Hartog Jacob Hamburger around the 1900s and is classified as an unbuffered/unbalanced crystalloid [1, 2]. Despite its name, it is not physiologically normal due to its higher chloride concentration, and zero strong ion difference (SID) compared to plasma. The SID of the plasma is approximately 40 mEq/l. As per the Stewart physiochemical approach, there is a net decrease in the plasma SID, after boluses of 0.9% NaCl, resulting in a non-anion gap metabolic acidosis. Non-anion gap acidosis engendered by 0.9% NaCl may lead to hyperkalemia, diminished renal perfusion and increased mortality. Sodium and chloride balance will be discussed in Chap. 23.
Buffered/Balanced Crystalloids
Buffered/balanced crystalloids are designed to mimic the composition of human plasma. The key differences between 0.9% NaCl and buffered/balanced crystalloid solutions are the presence of physiological or near-physiological amounts of chloride, a nearly physiological SID and also the presence of additional anions, such as lactate, acetate, malate and gluconate. These anions act as physiological buffers by generating bicarbonate on metabolism. Despite physiological similarities of these solutions to human plasma, presently there is no ideal balanced or physiologically “normal” crystalloid (Table 24.1).
Buffered/balanced crystalloids are commonly used as both resuscitation and maintenance fluid in the emergency room, intensive care units and during elective and emergency surgery. Various buffered solutions are not biologically equivalent due to a variable concentration of ancillary cations (sodium, potassium, calcium, magnesium) in addition to differences in buffering agents. Furthermore, a difference in osmolarity of various solutions is also an important practical consideration. As shown in Table-1, Ringer’s lactate (RL) and Ringer’s acetate (RA) are hypotonic solutions that can lead to post-resuscitation positive fluid balance, edema, weight gain and increase in intracranial pressure when used in larger volumes [3].
Sodium Lactate vs Sodium Acetate Solution
In the 1880s, British physiologist Dr. Sydney Ringer demonstrated the influence of crystalloids on the ex vivo beating of a frog’s heart [4, 5]. This fluid was known as Ringer’s solution. Ringer’s solution was modified in 1932 by American pediatrician Dr. Alexis Hartmann by adding sodium lactate to act as a buffering agent in an effort to combat acidosis [6, 7]. This fluid was popularized as sodium lactate or RL or Hartmann’s solution and may be considered the first balanced crystalloid. Lactated solution contains 2.7 mmol/l of calcium and and should not be mixed with blood or blood-related products. In fact, mixing calcium containing solutions with some antibiotics like ceftriaxone can cause the precipitation asinsoluble ceftriaxone calcium salt [8]. Use this recent reference for compatibility of various solutions with balanced crystalloids. PMID: 32401743.
There is an abundance of human studies comparing 0.9% saline with balanced solutions but data is scarce for the comparison of various balanced solutions. To compare acetate with lactate: acetate is metabolized widely and more rapidly than lactate and is not entirely dependent on hepatic metabolism and patient age [9]. Also, acetate solutions are less liable to bacterial contamination when compared with lactate in peritoneal dialysate solutions [10]. In contrast to lactate metabolism, acetate metabolism is preserved in severe shock [11]. Acetate is also more alkalinizing than lactate, which may confer benefits in treating acidemic patients requiring fluid resuscitation. Acetate does not affect glucose or insulin concentration unlike lactate, which is converted to glucose via gluconeogenesis and can cause significant hyperglycemia especially in patients undergoing major surgery [12, 13].
Hypoxia and hypotension can occur in patients with chronic kidney disease dialyzed with solutions containing acetate because of increased nitric oxide synthesis [14,15,16]. Kirkendol RL et al., first reported that sodium acetate produced a dose-related decrease in cardiac contractility and blood pressure in dogs [17]. Initial reports by Kirkendol RL et al. conflicted with his own research showing that a slow infusion of acetate failed to cause adverse haemodynamic effects [18]. Two decades later, Jacob et al., also examined the effect of acetate on myocardial energy metabolism on isolated perfused rat heart and reported a negative effect on cardiac contractility [19].
There were no human studies to support these findings until Schrander-vd Meer AM, demonstrated the haemodynamic effects of acetate in patients undergoing high-volume renal replacement therapy (RRT) [20]. He concluded that acetate-associated vasodilatation and negative inotropic effect led to haemodynamic instability in these patients.
In another crossover study on humans involving 12 patients undergoing hemo-diafiltration, Selby et al. demonstrated that exposure to acetate-free dialysate was associated with less deterioration in systemic haemodynamics, and less suppression of myocardial contractility [21].
In a recent study, RL and RA infusions were compared with regard to acid–base balance and haemodynamic stability in patients undergoing abdominal gynecologic surgery. Patients received a mean dose of 4054 ± 450 ml of either one or the other; there was no difference in terms of mean arterial blood pressure and norepinephrine requirements between the study groups. pH and serum HCO3- concentration decreased slightly but significantly only with RL [22].
Patients with liver disease pose a challenge to anaesthesiologists and intensivists; the fluid choice for resuscitation and maintenance is an important consideration. In a study of patients with cirrhosis undergoing general anaesthetic for elective surgery by Hatem A Attalla et al., RA was associated with significantly higher pyruvate levels and ketone bodies while lactate was higher in the RL group. pH, HCO3, base excess, liver function, blood glucose level and haemodynamic parameters were similar in both groups. These findings suggest that RA decreased the metabolic load to the liver and improved hepatic energy status in patients with liver dysfunction, so it may be more beneficial than RL as an intraoperative fluid [23].
In another study, Nakayama et al. studied the effect of RA and RL on intraoperative and postoperative haemodynamics, metabolism, blood gas and renal as well as liver function in patients undergoing hepatectomy. Intraoperative serum lactate levels increased significantly in both groups however, postoperatively, lactate levels were significantly higher in the RL group [24].
In another interesting study, RA was compared with RL to find its usefulness in patients with liver dysfunction. Acid–base balance, electrolytes and liver function showed no significant changes in any group. It was concluded that the status of liver dysfunction did not affect the metabolism of lactic acid and either RA or RL can be used as intraoperative maintenance fluid [25].
Hyperglycemia in the perioperative period results in increased morbidity and mortality [26]. Precipitating factors for hyperglycemia are stress response to surgery and anesthesia, intraoperative use of drugs, tissue damage and bleeding. There is a fear of using RL in the perioperative period because of the risk of lactate being converted to glucose especially in diabetic patients. A recent prospective, parallel group, observational study assessed the incidence of hyperglycemia, lactatemia and metabolic acidosis with the use of RL and RA in non-diabetic patients undergoing major head and neck free flap or abdominal surgery [27]. Intraoperative hyperglycemia was more frequent in RL group compared to RA group. RA group patients undergoing major abdominal surgery showed higher blood sugar compared to free flap surgery. When the duration of surgery exceeded 6 h, acetate-based solutions resulted in significantly higher lactate levels with progressive metabolic acidosis.
In conclusion, RL and RA are comparable in terms of haemodynamic stability and acid–base status. Longer surgery duration resulted in significantly higher lactate levels with progressive metabolic acidosis when using acetate-based fluids. RL and RA can both be used as intraoperative maintenance fluid in patients with liver dysfunction. Hyperglycemia was variable with different types of surgical procedures.
Acetate and Gluconate Buffered Solutions
This is an isotonic, buffered intravenous crystalloid solution with a physiochemical composition that closely reflects human plasma. Unlike RL, which contains calcium; acetate and gluconate-based buffered solution are calcium-free and therefore compatible with blood and blood components.
These solutions contain 23 mmol/L of gluconate. Little is known about the clinical significance of gluconate. Approximately 80% of gluconate is eliminated via renal mechanisms. As gluconate plays a role in the galactomannan antigenicity, patients receiving Plasma-Lyte®-148 (PL-148) may test false positive for the galactomannan antigen lasting up to 24 h [28, 29].
It is unclear if PL-148 will lead to an increase in unmeasured anions when given in large quantities. In an Australian study, TJ Morgan compared bicarbonate-based balanced fluid with PL-148 for cardiopulmonary bypass circuit priming. With the trial fluid, metabolic acid–base status was normal, whereas PL-148 triggered a surge of unmeasured anions persisting throughout bypass, and produced a slight metabolic acidosis without a clear clinical significance [30].
In a similar study by Davies et al, it was observed that when PL-148 was administered as a cardiopulmonary bypass pump-prime fluid, there were supra-physiologic plasma levels of acetate and gluconate when compared to a bicarbonate pump priming solution. The implications of supra-physiological gluconate and acetate levels remain undetermined [31].
The development of metabolic acidosis is well recognized during cardiopulmonary bypass. It is postulated that PL-148 will not lead to acidosis compared to RL as a priming fluid. In a prospective, double blind, randomized control trial on 22 patients undergoing cardiopulmonary bypass for coronary artery bypass surgery, Liskaser et al. compared Haemaccel-Ringer’s and PL-148 as a priming fluid for the development of acidosis [32]. All patients developed a metabolic acidosis and the decrease in base excess was the same for both primers, although with different mechanisms of acidosis viz hyperchloremic with Haemaccel-Ringer’s and increase in unmeasured anions (most probably acetate and gluconate) with PL-148.
For patients undergoing major liver surgery, use of lactate-free solutions may be beneficial. Shin WJ et al., compared the effects of RL and PL-148 on liver functions and serum lactate in living donors undergoing right hepatectomy. The lactate concentrations was significantly higher in the RL group than in the PL-148 group, 1 h postoperatively. In addition, albumin levels were significantly lower and the peak total bilirubin concentration and prothrombin time were significantly higher in the RL group. However, these changes did not persist beyond the first or second postoperative days [33].
A similar multicenter, prospective, double-blind randomized controlled trial by Weinberg L et al compared the effects of Hartman solution (HS) with PL-148 in patients undergoing major liver resection. Patients treated with HS were more hyperchloremic, hyperlactatemic and also lost more blood. Mean PT and aPTT were significantly lower and haemoglobin was higher, immediately after surgery in the PL-148 group. There were no significant differences in pH, bicarbonate, albumin and phosphate levels [34].
There is little data to compare various buffered solutions peri-operatively for renal transplantation. Normal saline is widely advocated as it is potassium free, however recent evidence suggests that balanced solutions may be more appropriate. In a double-blind study, the effect of different crystalloids on acid–base balance and early kidney function postrenal transplant was studied by Hadimioglu et al. Patients were randomized to three groups to receive either normal saline, RL or PL-148. There was a statistically significant decrease in pH and base excess and a significant increase in serum chloride in patients receiving saline during surgery. Lactate levels increased significantly in patients who received RL. No significant changes in acid-base balance or lactate levels occurred in patients who received PL-148 [35].
There are few studies that have monitored changes in cognitive functioning as a result of infusion of different buffered solutions. In a recent Indian study, Jigyasa Shahani et al. compared the cerebral-protective effects of RL and gluconate-based buffered solution (Kabilyte) in patients undergoing cardiopulmonary bypass. There was no significant difference between the preoperative and postoperative cognitive test scores in both groups [36].
In conclusion, RL and gluconate-based buffered solutions are comparable in terms of metabolic acid–base status and cognitive function in patients undergoing cardiopulmonary bypass. The surge of unmeasured anions remains clinically non-relevant. Use of gluconate-based buffered solutions does not appear to be of benefit in patients undergoing liver surgery.
Whether the use of balanced multi-electrolyte solution (PL-148) in preference to 0.9% sodium chloride solution (saline) in critically ill patients reduces the risk of acute kidney injury or death is uncertain. Simon Finfer et al compared the efficacy of PL-148 with saline as fluid therapy in the intensive care unit (ICU) for 90 days in a multicenter, prospective, doubleblind randomized controlled trial. Death within 90 days after randomization occurred in 21.8% in the PL-148 group versus 22.0% in the saline group (P=0.90), which was statistically non-significant. New renal-replacement therapy was initiated in 12.7% in the PL-148 group versus 12.9% in the saline group, which was also non-significant. The mean (±SD) maximum increase in serum creatinine level was 0.41±1.06 mg per deciliter (36.6±94.0 μmol per liter) in the PL-148 group and 0.41±1.02 mg per deciliter (36.1±90.0 μmol per liter) in the saline group, for a difference of 0.01 mg per deciliter. The number of adverse and serious adverse events did not differ meaningfully between the groups. Authors found no evidence that the risk of death or acute kidney injury among critically ill adults in the ICU was lower with the use of PL-148 than with saline [37].
Acetate and Malate Buffered Solutions
Malate is a citric acid cycle intermediate. It is a standard component in resuscitation and maintenance fluid therapy for a wide range of patients, e.g. sepsis, trauma, perioperative and critically ill patients. Data is insufficient regarding its plasma distribution, renal excretion and metabolism after intravenous injection except from a small number of animal studies. The liver is an important consumer of malate [38]; the kidneys also play an important role in malate metabolism.
Intraoperative fluid management continues to be a daily challenge in anesthetic practice. Various buffered solutions have different effects on acid–base status, electrolyte levels, coagulation and renal and hepatic function. A recent study by Morgan TJ et al., compared the influence of RL and malate buffered solution (Sterofundin) on acid–base changes, haemodynamics and readiness for extubation on 30 consecutive children undergoing scoliosis surgery. There was no statistically significant difference in the volume of infused fluid and changes in pH. The strong ion difference was decreased in both groups, though it normalized earlier with Sterofundin [39]. What about other primary outcomes?
In another recent study on children, RL was compared with Sterofundin in terms of intraoperative acid-base and electrolytes status. A total of 30 children aged between 1 and 13 years were randomized. In the RL group, the mean difference in pH between the baseline and end of surgery was statistically significant. The mean difference in base excess was similar in both groups [40].
A prospective, randomized, controlled trial assessed the effects of using Sterofundin or RL as the intraoperative fluid in patients undergoing major head and neck surgery with free flap reconstruction. Intraoperative lactate levels were significantly high in the RL group at 2, 4, 6, and 8 h. The pH was comparable between groups except at 8 h where the RL group had a significantly lower pH than the Sterofundin group. There was no significant difference between both groups in terms of sodium, potassium, chloride, bicarbonate and pCO2 levels [41].
In conclusion, RL and Sterofundin are comparable in terms of acid–base physiology and electrolytes in adults but Sterofundin is better in pediatric patients undergoing major surgery. Also, use of Sterofundin reduced lactate levels in comparison with RL in patients undergoing prolonged surgery.
There are no human studies comparing the four balanced crystalloids as resuscitation fluid; a single large study in animals was performed in the late 80 s. This compared the ability of four balanced crystalloid solutions (normal saline, RL, Plasma Lyte-A and Plasma Lyte-R) to prevent death after a fatal haemorrhage to simulate human exsanguination in unanesthetized swine. Swine were randomized to receive crystalloids at different percentages of replacement. The percentages of blood lost were replaced with 14% normal saline in 5 minutes, 100% normal saline in 20 minutes, and 300% normal saline, RL, Plasma Lyte-A and Plasma Lyte-R [42]. RL provided the best survival rate of 67% compared to 30% with Plasma-Lyte-A. After an analysis of arterial blood gas, lactate, acid-base, heart rate and aortic pressure measurements, it was concluded that RL is the superior crystalloid because of its decreased chloride load (compared to normal saline) and the absence of acetate or magnesium (compared to Plasma-Lyte-14).
Case Vignette
Mr. X, the patient in the vignette shows clinical signs of septic shock and the purpose of resuscitation is to improve tissue perfusion. The choice of fluid should be based on clinician preference. It is reasonable to resuscitate Mr. X with normal saline if the volume used is not large (1 to 2 L) otherwise balanced solutions are preferred.
Conclusion
It is unlikely that one balanced solution is better than another in this situation. Ideally, the choice of fluid for resuscitation/maintenance should be individualized qualitatively, e.g. a particular fluid for a specific type of surgery and quantitatively, e.g. an appropriate amount at the appropriate time and at the appropriate rate. Results from single-center studies should be viewed with caution before making any clinical decision based on these. There is a strong need for multi-center trials comparing various balanced solutions such as resuscitation and /or maintenance fluid.
Take Home Message
-
Buffers play a crucial role in maintaining acid–base balance in the body.
-
Different buffers have different characteristics and can be used in balanced solutions for different purposes.
-
Lactate, acetate, gluconate and malate are the most commonly used buffers in balanced solutions and can help prevent the development of hyperchloremic metabolic acidosis.
-
The choice of buffer in balanced solutions should be tailored to the patient’s specific clinical needs and conditions.
-
The optimal buffer for balanced solutions is still under debate, and further research is needed to determine the best choice in different clinical scenarios.
References
Hamburger HJ. Osmotischer Druck und Ionenlehre in den medicinischen Wissenschaften: Zugleich Lehrbuch physikalischchemischer Methoden. Wiesbaden: J. F. Bergmann; 1902.
Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27(2):179–88.
Tommasino C, Moore S, Todd MM. Cerebral effects of isovolemic hemodilution with crystalloid or colloid solutions. Crit Care Med. 1988;16:862–8.
Miller DJ. Sydney Ringer; physiological saline, calcium and the contraction of the heart. J Physiol. 2004;555(Pt 3):585–7.
Ringer S. Regarding the action of the hydrate of soda, hydrate of ammonia, and the hydrate of potash on the ventricle of the frog’s heart. J Physiol (London). 1880/82;3:195–202.
Lee JA. Sydney Ringer (1834–1910) and Alexis Hartmann (1898–1964). Anaesthesia. 1981;36(12):1115–21.
Hartmann AF, Senn MJ. Studies in the metabolism of sodium R-lactate. I. Response of normal human subjects to the intravenous injection of sodium R-lactate. J Clin Invest. 1932;11(2):327–35.
Murney P. To mix or not to mix – compatibilities of parenteral drug solutions. Aust Prescr. 2008;31:98–101.
Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979;64:708–13.
Richardson JA, Borchardt KA. Br Med J. 1969;3:749.
Kveim M, Nesbakken R. Utilization of exogenous acetate during canine haemorrhagic shock. Scand J Clin Lab Invest. 1979;39:653–8.
Akanji AO, Hockaday TD. Acetate tolerance and the kinetics of acetate utilization in diabetic and nondiabetic subjects. Am J Clin Nutr. 1990;51:112–8.
Arai K, Kawamoto M, Yuge O, Shiraki H, Mukaida K, Horibe M, Morio M. A comparative study of lactated Ringer and acetated Ringer solution as intraoperative fluids in patients with liver dysfunction. Masui. 1986;35:793–9.
Veech RL, Gitomer WL. The medical and metabolic consequences of administration of sodium acetate. Adv Enzym Regul. 1988;27:313–43.
Quebbeman EJ, Maierhofer WJ, Piering WF. Mechanisms producing hypoxemia during hemodialysis. Crit Care Med. 1984;12:359–63.
Amore A, Cirina P, Mitola S, Peruzzi L, Bonaudo R, Gianoglio B, Coppo R. Acetate intolerance is mediated by enhanced synthesis of nitric oxide by endothelial cells. J Am Soc Nephrol. 1997;8:1431–6.
Kirkendol RL, Pearson JE, Bower JD, Holbert RD. Myocardial depressant effects of sodium acetate. Cardiovasc Res. 1978;12:127–36.
Kirkendol PL, Robie NW, Gonzalez FM, Devia CJ. Cardiac and vascular effects of infused sodium acetate in dogs. Trans Am Soc Artif Intern Organs. 1978;24:714–8.
Jacob AD, Elkins N, Reiss OK, Chan L, Shapiro JI. Effects of acetate on energy metabolism and function in the isolated perfused rat heart. Kidney Int. 1997;52:755–60.
Schrander-vd Meer AM, Ter Wee PM, Kan G, et al. Improved cardiovascular variables during acetate free biofiltration. Clin Nephrol. 1999;51:304–9.
Selby NM, Fluck RJ, Taal MW, McIntyre CW. Effects of acetate-free double-chamber hemodiafiltration and standard dialysis on systemic hemodynamics and troponin T levels. ASAIO J. 2006;52:62–9.
Klaus F, Hofmann-Kiefer DC, Kammerer T, Jacob M, Paptistella M, Conzen P, Rehm M. Influence of an acetate- and a lactate-based balanced infusion solution on acid base physiology and hemodynamics: an observational pilot study. Eur J Med Res. 2012;17:21.
Hatem A, Attalla MD, Montaser S, Abulkassem MD, Khaled M, Abo Elenine MD. Alexandria J Anaesthesia Intensive Care. 2005;8(2)
Nakayama M, Kawana S, Yamauchi M, Tsuchida H, Iwasaki H, Namiki A. Utility of acetated Ringer solution as intraoperative fluids during hepatectomy. Masui Jpn J Anesthesiol. 1995;44:1654–60.
Isosu T, Akama Y, Tase C, Fujii M, Okuaki A. Clinical examination of acetated Ringer solution in patients with normal liver function and those with liver dysfunction. Masui Jpn J Anesthesiol. 1992;41:1707–13.
Tilak V, Schoenberg C, Castro AF 3rd, Sant M. Factors associated with increases in glucose levels in the perioperative period in non-diabetic patients. Open J Anaesth. 2013;3:176–85.
Balakrishnan S, Kannan M, Rajan S, Purushothaman SS, Kesavan R, Kumar L. Evaluation of the metabolic profile of ringer lactate versus ringer acetate in nondiabetic patients undergoing major surgeries. Anesth Essays Res. 2018;12:719–23.
Petraitiene R, Petraitis V, Witt JR, Durkin MM, Bacher JD, Wheat LJ, Walsh TJ. Galactomannan antigenemia after infusion of gluconate-containing plasma-Lyte. J Clin Microbiol. 2011;49:4330–2.
Hage CA, Reynolds JM, Durkin M, Wheat LJ, Knox KS. Plasmalyte as a cause of false-positive results for aspergillus galactomannan in bronchoalveolar lavage fluid. J Clin Microbiol. 2007;45:676–7.
Morgan TJ, Power G, Venkatesh B, Jones MA. Acid-base effects of a bicarbonate-balanced priming fluid during cardiopulmonary bypass: comparison with plasma-Lyte 148. A randomised single-blinded study. Anaesth Intensive Care. 2008;36:822–9.
Davies PG, Venkatesh B, Morgan TJ, Presneill JJ, Kruger PS, Thomas BJ, Roberts MS, Mundy J. Plasma acetate, gluconate and interleukin-6 profiles during and after cardiopulmonary bypass: a comparison of plasma-Lyte 148 with a bicarbonate-balanced solution. Crit Care. 2011;15:R21.
Liskaser FJ, Bellomo R, Hayhoe M, Story D, Poustie S, Smith B, Letis A, Bennett M. Role of pump prime in the etiology and pathogenesis of cardiopulmonary bypass-associated acidosis. Anesthesiology. 2000;93:1170–3.
Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558–64.
Weinberg L, Pearce B, Sullivan R, Siu L, Scurrah N, Tan C, Backstrom M, Nikfarjam M, McNicol L, Story D, Christophi C, Bellomo R. The effects of plasmalyte-148 vs. Hartmann’s solution during major liver resection: a multicentre, double-blind, randomized controlled trial. Minerva Anestesiol. 2015;81:1288–97.
Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264.
Shahani J, Saiyyed A. Comparison of Kabilyte® and Ringer lactate as prime solution on cognitive functions in patients undergoing cardiopulmonary bypass. Int J Sci Res(IJSR). 2017;6(7):1754–6.
Simon Finfer, Sharon Micallef, Naomi Hammond, et al. For the plus study investigators and the australian and new zealand intensive care society clinical trials group. Balanced multielectrolyte solution versus saline in critically ill adults. NEJM 2022;386(9):815–26.
Zander R. Fluid Management. 2nd ed. Melsungen: Bibliomed-Medizinische Verlagsgesellschaft mbH; 2009.
Sharma A, Monu Yadav B, Rajesh Kumar P, Lakshman S, Iyenger R, Ramchandran G. A comparative study of Sterofundin and Ringer lactate based infusion protocol in scoliosis correction surgery. Anaesth Essays Res. 2016;10(3):532–7.
Shariffuddin BAPP, Adeline C, Chinna K, Lucy C. A comparison of Sterofundin and Ringer’s lactate on intraoperative acid base and electrolytes status in children: a randomized controlled trial. Anaesth Critic Care Med J. 2018;3(1):000128.
Rajan S, Srikumar S, Tosh P, Kumar L. Effect of lactate versus acetate-based intravenous fluids on acid-base balance in patients undergoing free flap reconstructive surgeries. J Anaesthesiol Clin Pharmacol. 2017;33:514–9.
Traverso LW, Lee WP, Langford MJ. Fluid resuscitation after an otherwise fatal hemorrhage: I. Crystalloid solutions. J Trauma. 1986;26:168–75.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Garg, S.K. (2024). Balanced Solutions: Choice of Buffer. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_24
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)