Abstract
The waters of the United Arab Emirates (UAE) are home to a wide variety of shark and ray species, many of which are unique to the region and about which relatively little is known. Research efforts to date have focused primarily on identifying the species that occur locally and their importance to fisheries, but further research is required to understand their inherent biological and ecological traits. Decades of heavy fishing pressure and coastal development have impacted shark and ray populations to the extent that some, once common species, are now considered rare. Encouragingly, the UAE has adopted a National Plan of Action for the Conservation of Sharks to guide researchers and policy makers in their efforts to ensure that the nation’s shark and ray populations are effectively managed and conserved.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 What Exactly Are ‘Sharks’ and ‘Rays’?
The term ‘sharks and rays’ refers to a diverse group of aquatic vertebrates known scientifically as the elasmobranch fishes (Elasmobranchii). They differ from other fish groups in a variety of ways, but most notably in the possession of a skeleton that is composed entirely of cartilage (Klimley 2013). It is because of this trait that they are sometimes referred to as the ‘cartilaginous fishes’, while all other fish groups are collectively known as the ‘bony fishes’ (due to the presence of bone, to varying degrees, in their skeletons). However, the elasmobranchs differ from bony fish in a variety of other ways as well, which is hardly surprising given that this group has been evolving independently for over 400 million years. Indeed, the modern elasmobranchs are the living descendants of one of the earliest vertebrate lineages and, consequently, they have retained many ancestral characteristics (Grogan and Lund 2004).
Although a detailed exploration of elasmobranch anatomy and physiology is beyond the scope of this chapter, some of their distinguishing traits are worth emphasising. Externally, the most obvious difference is that elasmobranchs do not possess the relatively large, flexible, scales that are typical of most bony fish. Instead, their skin is covered with tiny placoid scales known as denticles, which give elasmobranch skin its characteristic sandpaper-like texture (Fig. 20.1). However, in some species the skin is largely devoid of scales, while in others the denticles are enlarged to form prominent thorn-like structures (Meyer and Seegers 2012). It is notable from an evolutionary perspective that denticles have the same basic structure as teeth, consisting of an interior pulp cavity surrounded by a hard mineralised material called dentine which, in turn, is overlain with enamel.
Also evident externally is the fact that elasmobranchs have multiple paired gill openings, as compared to the single pair of gill openings found on a bony fish. These paired gill openings range in number from five to seven, with the vast majority of species having five (Fig. 20.1). Species with six or seven pairs of gill openings represent more ancient branches of the elasmobranch evolutionary tree and are largely confined to the deeper regions of the ocean (Ebert et al. 2021; Last et al. 2016).
From a distance, sharks might appear to possess smooth skin, but on closer inspection their placoid scales are clearly evident (left). Another key difference between elasmobranchs and other fish groups is that they possess multiple, paired gill openings. These are typically found on the side of the head in sharks, as seen here (right), but on the underside of the head in batoids. Image credit: Aspas/shutterstock.com (left) and Stefan Pircher/shutterstock.com (right), used under license to S. Al Hamali
Perhaps the most notable difference between elasmobranchs and the vast majority of other fishes, is that they reproduce via internal fertilisation. Whereas most bony fish release their eggs and sperm into the surrounding water for fertilisation to take place outside the body, the elasmobranchs undergo a physical copulation, whereby the male deposits his sperm inside the female’s body (Pratt and Carrier 2011). This is achieved with copulatory organs known as claspers, which are scroll-like extensions of the pelvic fins. As claspers are only found on males, their presence or absence allows the gender of any elasmobranch to be ascertained easily.
Following copulation, the sperm that have been deposited in the female’s reproductive tract must undertake a lengthy trip through the uterus until they reach the oviducal gland; a structure that is unique to elasmobranchs and which performs a variety of tasks (Hamlett et al. 2005a). It acts initially as a receptacle for the sperm, where they can be stored for periods of days, months, or even years, until the female ovulates (Pratt 1993). It is thought that the oviducal gland is also the site of fertilisation, after which it secretes a protective covering around the egg (Hamlett and Koob 1999). The nature of this protective covering varies according to the reproductive strategy of the species in question, for the females of some species release the fertilised egg from the body and embryonic development is completed externally, while others retain the fertilised egg in the uterus for embryonic development to be completed within the female’s body (Carrier et al. 2004). In the case of the former, egg-laying species, the egg covering is usually tough and leathery (commonly called a “mermaid’s purse”), while in the latter, live-bearing species, it is thin and membranous. The reproductive output of egg-laying species is not constrained by the size of the female’s body, as she can continue to lay eggs for as long as there are viable sperm stored in the oviducal gland. However, these embryos will rely entirely on the yolk reserves in the egg to fuel their development until they hatch from the egg-case a number of months later. Consequently, they tend to be small in size and vulnerable to predation at the time of hatching. The females of live-bearing species can produce only as many embryos as their body can physically accommodate for the duration of the pregnancy. However, a variety of strategies have evolved by which the mother can pass additional nutrients to her embryos, so that they are not solely reliant on their yolk reserves (Hamlett et al. 2005b). So, although far fewer embryos are produced, they tend to be larger and less prone to predation at the time of birth. These aspects of elasmobranch reproductive biology have a large bearing on their varying abilities to withstand fishing pressure and habitat loss, and we will revisit this topic later in this chapter.
Returning to the question posed at the beginning of this section, why do we distinguish between two distinct groups of elasmobranchs, namely ‘sharks’ and ‘rays’? As in all matters related to taxonomy, the answer is complicated but, in essence, sharks have gill openings placed on the sides of the head, whereas rays have gill openings that are positioned on the underside of the head. While this might seem like an arbitrary difference, it does appear to reflect a clear genetic division within the elasmobranchs (Naylor et al. 2005). The sharks form a lineage that is usually referred to as the Selachii while the rays form a lineage known as the Batoidea. Although sharks vary in colouration and range in size from the diminutive dwarf lanternshark (Etmopterus perryi) that reaches a length of only 21 cm, to the gigantic whale shark (Rhincodon typus) that reaches a length of almost 20 m, their body form is relatively well conserved (with a few exceptions) (Ebert et al. 2021). On the other hand, rays are a much more diverse group that encompasses a variety of body forms, including the egg-laying skates, the live-bearing stingrays, guitarfishes and sawfishes, among others (Last et al. 2016).
2 Ecological Importance
Elasmobranchs are found throughout the marine environment, ranging from shallow inshore areas to the deepest depths of the ocean. The bull shark (Carcharhinus leucas), is famous for being able to move between marine and freshwater environments (Thorson 1971), but a number of other shark and ray species are also euryhaline (capable of withstanding wide fluctuations in salinity), while a small number of ray species are restricted to freshwater (Lucifora et al. 2015). Regardless of the environment in which they are found, all elasmobranch species are predatory in nature (Wetherbee and Cortés 2004). Prey preferences vary from species to species and are largely informed by lifestyle. Demersal species, i.e. those that live close to the seabed, tend to have diets dominated by a variety of bottom-dwelling invertebrates such as crabs and polychaete worms, although fish, squid and octopuses may also be consumed. Pelagic species, i.e. those that live higher in the water column, usually have diets that are dominated by fish; although, it is worth pointing out that the two largest shark species in the world, the whale shark and the basking shark, are filter-feeders whose diets consists mostly of zooplankton (Wetherbee and Cortés 2004). The routine consumption of marine mammals, sea turtles and seabirds is the preserve of a small number of shark species, such as the great white shark (Carcharodon carcharias) and the tiger shark (Galeocerdo cuvier).
It is common to see elasmobranchs referred to as ‘apex predators’, meaning that they occupy the highest trophic (i.e. feeding) level of the food web that they are part of (Wallach et al. 2015). However, this is a somewhat simplistic and misleading representation of their ecological role. After all, we are talking about a diverse group of animals of varying sizes, lifestyles and dietary preferences. For example, the Arabian carpetshark (Chiloscyllium arabicum) is a common demersal shark in the coastal waters of the UAE that feeds mostly on small invertebrates and grows to a maximum size of around 90 cm (Ebert et al. 2021). An animal of this size is incapable of feeding on large fish, marine reptiles or mammals, but is itself likely to be an important prey item for larger predators (Fig. 20.2). On the other hand, the tiger shark can reach lengths well in excess of 500 cm and is unlikely to be a common prey for any other species—at least by the time it reaches adult size. It also feeds on a wide variety of species, including other sharks, sea turtles and marine mammals (Heithaus 2001). So, whereas the latter can truly be considered an apex predator, the former is most certainly not. Indeed, it is a relatively small number of large-bodied shark species that deserve the apex predator moniker, while most other sharks and batoids are more accurately described as ‘mesopredators’, i.e. they occupy intermediate trophic levels.
Nonetheless, both apex predators and mesopredators play important roles in the structuring and functioning of food webs, and, as such, factors that impact their abundance in an ecosystem can have far reaching consequences (Pauly et al. 1998). The most publicised of these is the so-called ‘trophic cascade’, whereby changes in the abundance of a species in one trophic level cascades through all trophic levels down to the primary producers (e.g. marine plants and algae) (Terborgh et al. 2010). It is hardly surprising then, that numerous studies have linked wide-ranging negative ecological consequences to the overfishing of elasmobranch species (Barley et al. 2017; Myers et al. 2007).
The importance of elasmobranchs to food web dynamics can also extend across multiple ecosystems. As we will see later in this chapter, some shark and batoid species inhabit what are termed ‘nursery grounds’ during the early stages of life, and these are typically shallow, inshore areas where food is abundant and potential predators are less common (Heupel et al. 2007). The fact that they are feeding in these productive inshore areas during their early years, before moving out to deeper coastal areas or the open ocean, where they may fall prey to larger predators, means that they are effectively transferring nutrients and energy from one place to another. In other words, they form a trophic link between what might otherwise be largely distinct ecosystems (Osgood and Baum 2015; Sievers et al. 2019). While such a role is not limited to elasmobranchs, their sheer size elevates their importance in the process.
3 Sharks of the UAE
It would be reasonable to expect that this section of the chapter might begin with a straightforward statement declaring the number of shark species that have been recorded in UAE waters. Alas, there are two major complicating factors in this regard. The first is that, compared to many other parts of the world, relatively little scientific research was undertaken around the Arabian Peninsula until a few decades ago. Consequently, the field of marine research in the region is still quite young and there is a distinct lack of historical data to consider in the construction of local species checklists. The second complicating factor is that the world of taxonomic research in general has undergone something of a revolution in recent times, thanks to the advent of DNA barcoding (Savolainen et al. 2005). This has resulted in a huge increase in scientific publications that challenge pre-existing notions on species’ identities and relationships (DeSalle and Goldstein 2019)—particularly in geographic regions or with taxa that had already received limited taxonomic attention. Elasmobranchs in the Arabian region tick both of these boxes.
Keeping these caveats in mind, the most recent global checklist of shark species shows 47 (from 15 families) as possibly occurring in UAE waters (Ebert et al. 2021). These range in size from the Arabian carpetshark at 90 cm in length, to the world’s largest fish, the whale shark, that reaches up to 20 m in length. Of course, not all of these species can be considered common in UAE waters; indeed, definitive evidence for the local occurrence of a number of these species is lacking. To date, some 31 species have actually been confirmed in UAE waters, including zebra sharks (Stegastomatidae), whale sharks (Rhincodontidae), houndsharks (Triakidae), weasel sharks (Hemigalidae), requiem sharks (Carcharhinidae) and hammerhead sharks (Sphyrnidae). Of these, it is the requiem sharks that are by far the most common, with this family including large-bodied species such as the blacktip shark (Carcharhinus limbatus) and bull shark, and a wide variety of small-bodied species such as the milk shark (Rhizoprionodon acutus) and sliteye shark (Loxodon macrorhinus) (Box 20.1). A tentative checklist of shark species in UAE waters is provided at the end of this chapter.
Box 20.1 Commonly Misidentified Species in the UAE
When most people think of a shark, what they usually picture is grey and torpedo shaped, with the characteristic triangular dorsal fin. This is the typical appearance of a particular family of sharks—the requiem sharks (Carcharhinidae). This is one of the largest shark families and many of the species that it contains look very similar to each other.
This is particularly true of those species that commonly exhibit dark fin tips. To the uninitiated, it might seem reasonable to assume that a grey-coloured shark with dark fin tips is a ‘blacktip shark’. Alas, the situation is more complicated than this. A number of different species fit this general description including, of course, the actual blacktip shark. To complicate the matter further, the darkness of some or all fin tips can fade with age in some species. In UAE waters, the species that are most likely to be confused in this regard are the aforementioned blacktip shark, the spinner shark (Carcharhinus brevipinna) and the graceful shark (Carcharhinus amblyrhinchoides). Another species, the blacktip reef shark also has very prominent dark markings on its fins; however, whereas the former three species are all large-bodied and predominantly grey in colour, the latter doesn’t grow much larger than 1.5 m in length and its skin has more of a brownish-grey hue.
Other local elasmobranch species that look very similar to each other are the bull shark and pigeye shark (Carcharhinus amboinensis); the hooktooth shark (Chaenogaleus macrostoma) and the slender weasel shark (Paragaleus randalli); the cowtail ray (Pastinachus ater) and the broad cowtail ray (Pastinachus sephen); the leopard whipray (Himantura leoparda) and the coach whipray (Himantura uarnak).
The shark species that are most likely to be encountered by SCUBA divers and snorkelers in the UAE are the zebra shark (Stegostoma fasciatum) and the blacktip reef shark (Carcharhinus melanopterus), both of which associate with coral reefs and rocky outcrops (Fig. 20.3). Neither species poses a threat to humans as long as they are given a wide berth and not harassed. Zebra sharks will spend time lying on the seabed and can sometimes be easily approached, leading to a temptation to touch and stroke them. However, this type of behaviour is to be avoided as it can cause stress to the animal and, despite their generally docile nature, they can bite when provoked (Compagno 1984).
4 Rays of the UAE
As mentioned previously, ‘rays’ is the common term applied to the Batoidea, and this includes a wide variety of what are mostly dorso-ventrally depressed (i.e. they possess relatively wide, flat bodies) lineages. It is because of this body shape that they have come to be referred to as ‘flat sharks’ in recent parlance. The waters of the UAE are home to a diverse array of batoid species including sawfishes (Pristidae), wedgefishes (Rhinidae), guitarfishes (Rhinobatidae), giant guitarfishes (Glaucostegidae), butterfly rays (Gymnuridae), stingrays (Dasyatidae), eagle rays (Myliobatidae), pelagic eagle rays (Aetobatidae), cownose rays (Rhinopteridae) and devil rays (Mobulidae) (Last et al. 2016). Estimating the number of individual batoid species that occur in UAE waters is even more difficult than for sharks, as there is a great deal of uncertainty around the identities of numerous species (Naylor et al. 2012) (Box 20.2). In fact, it seems likely that many of the batoids that occur in UAE waters are either cryptic species (i.e. they look very similar to a well-known species from elsewhere but are in fact genetically distinct) or are a species complex (i.e. what appears to be a single species is actually two or more genetically distinct species). Unravelling this taxonomic tangle is a work in progress, but a tentative list of batoids in UAE waters is also provided at the end of this chapter.
Whereas the majority of shark species found in UAE waters are at least reasonably active swimmers, batoids more commonly spend extended periods lying on the seabed. This is particularly true of the guitarfishes, stingrays and butterfly rays, and it is species from these families that SCUBA divers and snorkelers in the UAE are most likely to encounter. However, the eagle rays, pelagic eagle rays, cownose rays and devil rays are all active swimmers, and it is species from these families that are most likely to be encountered near the surface (Fig. 20.4).
Box 20.2 When one species becomes four
If you were to consult a species guide from only a few years ago, you would likely find among its pages a species of stingray with white spots on its skin and black and white bands along its tail, known as the whitespotted whipray (Himantura gerrardi). Researchers had long thought that this might by a species complex consisting of two or more distinct species, and with the help of genetic analyses these suspicions turned out to be correct. The erstwhile H. gerrardi is now known to be four distinct species, namely Maculabatis gerrardi, Maculabatis bineeshi, Maculabatis arabica and Maculabatis randalli (Last et al. 2016).
Whenever a new species is described, it can take some time to determine the extent of its geographic distribution, and such is the case with these whiprays. Although they can all be found within what was H. gerrardi’s reported distribution (Indo-West Pacific), they don’t each necessarily occupy that whole range. Maculabatis gerrardi seems to have the broadest distribution of the four, occurring throughout the Indo-West Pacific including the Gulf of Oman. Maculabatis bineeshi has so far only been recorded from Indian waters and does not appear to occur in the Gulf of Oman or Arabian Gulf. Maculabatis randalli appears to be limited to the Arabian Gulf, possibly including the UAE’s Arabian Gulf coast, while Maculabatis arabica had, until recently, been limited to the west coast of India. However, it has since been documented from the UAE’s Arabian Gulf (Alhameli et al. unpublished data) and Gulf of Oman (Henderson 2020) coasts.
5 A Need for Nurseries: The Importance of Shallow, Inshore Habitats to Newborn Sharks and Rays
A nursery habitat, as the name suggests, is one that is utilised by newborn and juvenile sharks and rays. However, there is more to the concept than simply identifying an area where the young animals happen to occur. It is generally accepted that, in order to be considered a nursery area, it must meet the following criteria: Newborns and young juveniles are more common in the area under consideration than in other areas; they remain in, or return to, the area for extended periods; the area is repeatedly used across years (Heupel et al. 2007).
Nurseries tend to occur in productive, inshore areas, thereby providing the young sharks and rays with abundant feeding opportunities. Clearly, they play an extremely important role in the life cycles of species that use them, and anything that impacts the health and integrity of these areas can disrupt the populations that depend on them (Courrat et al. 2009).
In order to visualise how nurseries are used by elasmobranchs, we can look at the classic example of the lemon shark (Negaprion brevirostris), the reproductive biology and ecology of which has been studied in detail by researchers in the western Atlantic Ocean for a number of decades (e.g. Chapman et al. 2009; Correa et al. 1995; Feldheim et al. 2002; Gruber et al. 1988; Kessel et al. 2013). Pregnant females move into shallow coastal areas adjacent to mangrove forests in early summer, where they each proceed to give birth to between 4 and 17 pups, depending on the size of the female. Once birthing is complete, the female leaves the area and moves back out to deeper waters. The neonates, which measure around 60 cm in length, will remain in these very shallow waters for the first few years of their life. As they grow, they gradually increase the area that they utilise, eventually moving away from the mangroves into deeper sand flats and seagrass areas, eventually joining the adult population offshore or around coral reefs (Morrissey and Gruber 1993).
Many of the elasmobranchs that occur in UAE waters follow a similar life cycle as the lemon shark, including the closely related and almost identical sicklefin lemon shark (Negaprion acutidens). Archive footage from the BBC filmed during the early 1970s and available online shows Emirati fishers following large schools of these juveniles around the mangrove forests in Khor Faridah, Abu Dhabi. Alas, this species has been heavily fished and the population has declined to the extent that these large aggregations of juveniles are no longer common; however, the nurseries remain important for many other shark and ray species (Fig. 20.5). Moreover, scientists are still uncovering new and fascinating aspects of elasmobranch reproductive biology, some of which have dramatically altered our understanding of how these animals respond to adverse conditions (Box 20.3).
Box 20.3 Zebedee’s Virgin Births
In 2007, a zebra shark in the Burj Al Arab aquarium in Dubai laid 30 eggs, of which three contained embryos (Robinson et al. 2011). This, in itself, is not particularly unusual, as captive egg-laying species commonly produce unfertilised eggs (possibly as a means of preventing oocyte accumulation in the ovary). The interesting aspect of this story is that the shark, named Zebedee, had never been in contact with a male of her species during her adult life. Sharks, like all vertebrates, reproduce sexually, whereby a male sperm unites with a female ovum to produce a zygote that divides to become an embryo. So, without access to sperm from a male zebra shark, how could Zebedee have produced these embryos? What’s more, she went on to do the same thing in subsequent years!
The aquarium staff compared Zebedee’s DNA with that of the embryos and concluded that she had given birth through a process known as parthenogenesis, i.e. virgin birth. Although producing offspring in this manner is not unknown among vertebrates, it is extremely rare. The process has also been documented elsewhere in other elasmobranch species, including the bonnethead shark (Sphyrna tiburo), blacktip shark, white-spotted bamboo shark (Chiloscyllium plagiosum), smalltooth sawfish (Pristis pectinata) and whitespotted eagle ray (Aetobatus narinari) suggesting that it may not be all that uncommon in elasmobranchs. Indeed, it seems likely that at least some temporally unusual elasmobranch births in captivity that were originally attributed to female sperm storage, may have been due to parthenogenesis (Fig. 20.6).
6 Movements and Migrations: Lessons Learned from Tagging, Tracking and Genetic Studies
The most extensive elasmobranch tagging/tracking study in the region to date has been on whale sharks, using a combination of satellite-linked tags and photo-identification of individuals based on skin spot patterns (Robinson et al. 2017). Despite the fact that whale sharks are known to be a wide-roaming species, those in the Arabian Gulf were observed to remain there for extended periods, although movement between the Arabian Gulf and Gulf of Oman was also evident. Interestingly, the sharks aggregated at specific feeding sites during the summer but became more dispersed during winter, indicating seasonality in their habitat preference. Moreover, specific individuals were noted to return to the same feeding sites during each of the 5 years for which the study ran.
The movements of one shark in the study were particularly impressive, having travelled from the Al Shaheen gas field off Qatar to the coast of Somalia over the course of 37 days, a trip of some 2644 km. This was the only shark in the study to have moved beyond the Gulf of Oman. It was also one of the largest sharks to be tagged during the study, and is thought to have been a pregnant female. Although further studies are required to investigate whale shark movements over a longer timeframe, this points to the possibility that the sharks may utilise the Arabian Gulf and Gulf of Oman during specific life stages. It is further supported by the fact that small juveniles (<4 m in length) and large adults (>10 m in length) have not been observed in the region.
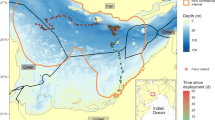
One other study has deployed satellite tags on sharks in the Gulf of Oman, with mixed results (Henderson and Reeve 2014). A bignose shark (Carcharhinus altimus) tagged off Muscat in 2011 moved approximately 97 km southward along the coastline over a period of 5 days. Unfortunately, the satellite track after this period is basically a straight line to the port of Al Quriyyat, indicating the shark was captured by fishermen and taken ashore. However, a sicklefin lemon shark tagged during the same study provided more extensive data. This shark was tagged off the Gulf of Oman coast of the Musandam peninsula in February 2011 and transmitted its archived data in May 2011. During this three-month period, it moved through the Strait of Hormuz into Iranian waters before moving westward through UAE waters, eventually ending up near Das Island. The total distance travelled by the shark was 578 km, in just 89 days (Fig. 20.7).
This map shows the path followed by a sicklefin lemon shark that was tagged with a pop-up satellite archival tag (PSAT) outside the Arabian Gulf, off the Musandam peninsula in 2011. The tag transmitted its data 3 months and 578 km later, when the shark was close to Das Island, Abu Dhabi (Source: A. Henderson, unpubl. data)
A more recent investigation into elasmobranch habitat use along the coast of Abu Dhabi has found an area near Ras Ghurab Island that seems to be of particular importance to two critically endangered batoids—the Pakistan whipray and the halavi guitarfish (Al Hameli et al. unpublished data). These species were considerably more abundant in this area than in the surrounding waters, and satellite tags that were deployed on some individuals indicate that they remain in the area for periods of at least several months.
While physically tagging and tracking marine animals has, historically, been the mainstay of understanding their movement patterns, advances in genetic analyses now facilitate researchers in the investigation of population structure and connectivity using DNA sequences. One such study assessed the population genetics of four shark species that occur around the Arabian Peninsula, from the Arabian Gulf to the Red Sea (Spaet et al. 2015). This included large-bodied species (blacktip shark and scalloped hammerhead shark), a medium-bodied species (spottail shark) and a small-bodied species (milk shark). At the outset of the study, it was assumed that each of the large-bodied species most likely formed a continuous population around the Arabian Peninsula, given their size and mobility, while the medium- and small-bodied species would be more likely to form multiple, smaller, discreet populations. What the study actually found is that there are no contemporary barriers to gene flow around the Arabian Peninsula in any of the study species. In other words, there are no population divisions around the Arabian Peninsula in any of the four species; they are each a pan-regional population.
Taken together, these studies tell us two important things. Firstly, the fact that individual animals commonly move across international boundaries and that populations are spread across multiple national territories complicates conservation efforts. Individual animals will be exposed to different fisheries and levels of protection as they move from one jurisdiction to another, and high levels of exploitation in one area are likely to impact whole populations. Consequently, there is a need for cooperative fisheries management in the region—after all, there is no point in one country have highly effective management measures in place if activities in other countries have a detrimental impact on those same populations. Secondly, the fact that some species, or particular life stages, might be highly dependent on habitats in specific locations, means that there is an onus on each country to identify and protect these areas.
7 Sharks and Rays in Emirati Culture
Traditionally, there are a number of gears and techniques used by Emirati fishers to target elasmobranchs, depending on the fishing area. In inshore shallow waters, particularly around islands, large meshed gill nets (leikh) 2–4 m deep and 100 m long with a mesh size of up to 30 cm were used to target sharks. Fishers usually deployed the nets overnight around particular areas where sharks were known to occur. Another gear type used in shallow waters is al sakkar, a temporary tidal barrier net usually 2 m deep and 500–1000 m long. Although based on the location and season, lemon sharks and rays can be commonly caught with this technique. Larger sharks, guitarfish and wedgefish were hunted using traditional wooden spears (oumlah) tied to a float that would tire the wounded fish and help in retrieving the catch. For offshore waters, longlines (manshallah) are usually deployed to target sharks from commercially registered dhows locally known as lanj (12–20 m long traditional vessels made from wood or fiberglass, that spend up to 10 days at sea).
Emirati fishers have traditional names for many local shark and ray species, with this sometimes extending to having multiple names for the same species depending on its size. Although fishers interviewed by the authors have expressed a preference for juvenile sharks, as the flesh becomes tougher with age, a wide variety of species and sizes have traditionally been caught. Accordingly, they have witnessed a great decline in some shark and ray populations within the areas they have historically fished, leading many to stop targeting elasmobranchs. One fisher pointed out that, in the past, he would encounter or catch between 7 and 10 elasmobranchs every other day, including juvenile blacktip sharks, whitecheek sharks, wedgefish, guitarfish and hammerhead sharks, but this is no longer the case. Of particular concern is that less than 20 years ago, wedgefish could be encountered on a daily basis, whereas now they might be seen only once or twice per year. When asked to quantify the decrease in local elasmobranch populations, the general consensus was a drop of approximately 50%, beginning in the early 2000s.
The traditional local utilisation of sharks and rays has been primarily for their meat. Dried and salted meat (ouwal) is consumed throughout the year, while fresh meat is used in a number of specific dishes such as jsheed. Liver oil from sharks and rays was also a valuable commodity at one time, as it was used to waterproof the hulls of wooden vessels. However, although still occasionally used in this manner, the advent of fibreglass vessels and synthetic sealants for wood, means that this is no longer common practice.
8 Threats to Shark and Ray Populations
There are two broad anthropogenic threats to shark and ray populations—indeed, to marine life in general. The first is overfishing, i.e. the removal of too many individuals from the population. The second is through habitat destruction or degradation.
Unfortunately, elasmobranchs have been heavily fished throughout the region and many species have experienced notable declines (Henderson et al. 2008; Jabado et al. 2014a; Jabado and Spaet 2017; Moore 2012). Shark and ray flesh has been utilised in local dishes for generations and this has resulted in targeted fisheries throughout the Arabian Gulf and neighbouring bodies of water. However, the level of exploitation underwent a dramatic increase in recent decades due to the demand for shark fin in the Far East and advances in fishing technology. Whereas the animals were once caught for their flesh and landed whole, the high value of shark fin meant that it became more profitable to remove the fins at sea and dump the rest of the carcass, so that the boat could be filled with the more valuable fins (Fig. 20.8). Thankfully, many countries eventually introduced legislation that prohibited the practice of finning at sea and required the animals to be landed whole. However, considerable damage was already done by that stage, and the on-going fisheries depleted the populations further (Box 20.4).
Even fins from small sharks are valuable to the shark fin market. Here, fins from small species such as the milk shark and slit-eye shark, as well as from juveniles of larger species such as the blacktip shark and scalloped hammerhead shark are being sun-dried prior to export to the Far East. Photographer: Aaron Henderson
Box 20.4 Where Did All the Sawfish Go?
The sawfishes (Pristidae) are large, shark-like batoids (Fig. 20.9). Although they possess an elongate body, the head is notably flattened and the gill slits are located on the underside—a defining feature of batoids. However, it is the long, toothed rostrum that is the most characteristic feature of the sawfishes and, of course, this is what gives them their name.
Written and photographic evidence indicates that sawfishes were abundant and widespread in the Arabian region up to the 1960s, but records thereafter are sparse, especially since the 1980s (Moore 2015). Although they have been a valuable commodity since the mid-nineteenth century, it seems that their precipitous decline coincided with the widespread availability of nylon gillets, in which their rostra become easily entangled. Although there are occasional, anecdotal reports of encounters with sawfish by UAE fishers, it seems that these animals are now incredibly rare, not just in the UAE but throughout the region.
Sawfish were once common in UAE waters but are now extremely rare throughout the region. Image credit: Shaun Wilkinson/shutterstock.com, used under license to S. Al Hameli
Whereas the impacts of fisheries on shark and ray populations are straightforward, human activities that alter habitat quality can be less obvious. It is easy to see why coastal development and construction projects might not be commonly thought of as a threat to mobile marine animals such as sharks—after all, there is a whole ocean there for them to use. However, as mentioned previously, many species rely on shallow, inshore habitats for their nursery grounds, and the loss of these areas can impact the reproductive ecology of the species in question. The knock-on effect is that recruitment—the addition of new juvenile individuals to the population—is reduced, further limiting the ability of these species to withstand any level of fishing pressure.
Of course, it is not just the complete loss of habitat that is of concern. Anything that influences water quality can alter ecosystem functioning and throw food webs out of kilter, thereby impacting all species that depend on these ecosystems (Palumbi et al. 2009). Eutrophication and the accumulation of pollutants can be particularly pronounced in coastal areas due to effluent discharge and surface run-off, further threatening the viability of inshore nursery grounds (Paerl 2006). Moreover, the Arabian Gulf is a semi-enclosed sea with a slow rate of water turnover, and this promotes the accumulation of pollutants on a broader scale (Naser 2013). As in the case of fisheries management, the management of water quality within the Arabian Gulf requires a collaborative approach.
Lastly, we must consider the potential impacts of climate change on local elasmobranchs. The Arabian Gulf is already the hottest sea in the world, and anything that might drive temperatures up is a matter for concern (see Chap. 4). Apart from the potential effects of temperature on elasmobranch physiology (Osgood et al. 2021), there is an inverse relationship between water temperature and dissolved oxygen. In other words, as water heats up, it contains less oxygen and, as with most marine animals, elasmobranchs depend on dissolved oxygen for respiration. This is cause for concern, as studies have already shown that low oxygen conditions are occurring around inshore reefs during summer (de Verneil et al. 2021).
However, there is another aspect of climate change that is of major concern in the marine environment: ocean acidification. Although a description of the process itself is beyond the scope of this chapter, ocean acidification is the term ascribed to the gradual decrease in ocean pH associated with the increase in atmospheric carbon dioxide. Its impact on marine life is far reaching, having consequences that are both direct and indirect, acute and gradual. Of immediate concern are the potential impacts of ocean acidification on the developmental biology of elasmobranchs, particularly in egg-laying species, where the developing embryo is exposed directly to environmental conditions (Johnson et al. 2016).
9 Data Requirements for the Effective Management of Shark and Ray Populations
There are three main groups of data that are of use in the management and conservation of exploited marine species. These consist of a species’ inherent biological traits (growth rate, size at maturity, reproductive output etc), its ecological characteristics (distribution, habitat requirements, movement patterns etc), and data relating to the exploitation of the species (capture data, landings data, fishery operational data etc). Where all of these data are available, they can be combined to model the population under various scenarios and levels of exploitation, thereby allowing policy makers to implement the most effective management and conservation measures. Typically, these consist of closed fishing seasons, areas in which fishing is temporarily or permanently prohibited, gear restrictions, catch limits, size limits, and so on (Hart and Reynolds 2002). However, if the data on which the management decisions are based are incomplete or inaccurate, the value of the management measures will be limited, and possibly even detrimental to the management goals.
The UAE, like most countries in the region, suffers from a paucity of biological, ecological and fisheries data regarding local elasmobranchs, and this creates numerous barriers to the formulation and implementation of effective management and conservation measures. As in many parts of the world, elasmobranch landings in the UAE are not identified to species; instead, they are broadly categorised as ‘shark’ or ‘ray’. Critically, this means that there is no reliable information available regarding the extent to which fishing activities are impacting any one species. On top of this, locally-derived biological and ecological data are few and far between, even for common species. Although there are published data for many of the UAE’s elasmobranch species from elsewhere in their respective ranges, these must be treated with caution. Elasmobranch growth rates, sizes at maturity, reproductive cycles, migration patterns etc. can vary with geographic location (Bradley et al. 2017), as most of these traits are intricately tied to the prevailing environmental conditions. Hence, data collected from the population under consideration are infinitely more valuable to policy makers than comparable data from a population that is far removed (Fig. 20.10).
10 Current National Management Measures
Under the UAE Ministerial Decree No. 43 for the Year 2019 on Regulating the Fishing and Trading of Sharks, there are nine articles aimed at the conservation and sustainable fishing of sharks and rays. The legislation states that registered lanj boats are permitted to fish for sharks and rays between July to February, with fishing banned between March and June. During the permitted fishing season there are limitations on the number and size of hooks that can be used (no more than 100 non-stainless steel circle hooks with a maximum size of 12/0), as well as the type of fishing nets that can be used (those constructed from nylon threads are banned).
The legislation also prohibits the capture of species listed on Appendix I or II of the Convention on the International Trade in Endangered Species of Wildlife and Flora (CITES) or on Appendix I of the Convention on the Conservation of Migratory Species of Wild Animals (CMS) at any time. Similarly, Federal Law No. 23 (and its Executive By-Laws) for the Year 1999 on the Exploitation, Protection and Development of Living Aquatic Resources also affords some species complete protection. In all of these cases, where a prohibited species is caught, or a permitted species is caught during the closed season, it should be released alive. When this is not possible, the carcass should be handed over to the competent local authority.
In addition to enacting specific legislation to protect elasmobranchs in UAE waters, the government has also followed the advice of the Food and Agricultural Organisation of the United Nations (FAO) in developing a National Plan of Action for the Conservation of Sharks (NPOA-Sharks). This document outlines the knowledge gaps and data requirements pertinent to the sustainable exploitation of shark and ray species and, therefore, provides guidance to researchers and policy makers in their efforts to ensure that the nation’s shark and ray populations are effectively managed and conserved (Anon 2018).
11 Conclusions
A diverse array of elasmobranchs species have been recorded in UAE waters, but years of heavy exploitation and habitat loss have impacted these populations, and many species are now extremely rare. However, all is not lost. Scientific interest in elasmobranchs is at an all-time high, and conservation concerns have been acknowledged by relevant authorities through the development of stronger fishing and trade regulations. It is important that this momentum is not allowed to fade. More research is required to better understand the biology, ecology and status of local elasmobranchs, while additional conservation regulations are required to help these populations to recover and ensure a bright future for this natural, and national, treasure.
12 Recommended Readings
For readers interested in learning more about the biology of sharks and rays, The Biology of Sharks and Their Relatives (Vol. 1 and 2), edited by J. C. Carrier et al. and published by CRC Press, is a good starting point. Those with a particular interest in elasmobranch taxonomy can access the extensive (and free) online Chondrichthyan Tree of Life at https://sharksrays.org .
References
Anon (2018) The UAE National Plan of action for the Conservation & Management of sharks. UAE Ministry of Climate Change & Environment
Barley SC, Meekan MG, Meeuwig JJ (2017) Species diversity, abundance, biomass, size and trophic structure of fish on coral reefs in relation to shark abundance. Mar Ecol Prog Ser 565:163–179. https://doi.org/10.3354/meps1198
Bradley D, Conklin E, Papastamatiou YP, McCauley DJ, Pollock K, Kendall BE, Gaines SD, Caselle JE (2017) Growth and life history variability of the grey reef shark (Carcharhinus amblyrhynchos) across its range. PLoS One 12:e0172370. https://doi.org/10.1371/journal.pone.0172370
Brown JNB (1992) Whale shark Rhincodon Typus (Smith, 1929). Tribulus 2:22
Carrier JC, Pratt HL, Castro JI (2004) Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Baton Rouge, pp 269–286
Chapman DD, Babcock EA, Gruber SH, Dibattista JD, Franks BR, Kessel SA, Guttridge T, Pikitch EK, Feldheim KA (2009) Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol Ecol 18:3500–3507. https://doi.org/10.1111/j.1365-294X.2009.04289.x
Compagno LJV (1984) Part 1: Hexanchiformes to Lamniformes. In: Sharks of the world: an annotated and illustrated catalogue of shark species known to date, FAO species catalogue, vol 4. FAO, Rome
Correa J, De Marignac J, Gruber S (1995) Young lemon shark behaviour in Bimini lagoon. Bahamas J Sci 3:2–8
Courrat A, Lobry J, Nicolas D, Laffargue P, Amara R, Lepage M, Girardin M, Le Pape O (2009) Anthropogenic disturbance on nursery function of estuarine areas for marine species. Estuar Coast Shelf Sci 81:179–190. https://doi.org/10.1016/j.ecss.2008.10.017
de Verneil A, Burt JA, Mitchell M, Paparella F (2021) Summer oxygen dynamics on a southern Arabian Gulf coral reef. Front Mar Sci 8:1676. https://doi.org/10.3389/fmars.2021.781428
DeSalle R, Goldstein P (2019) Review and interpretation of trends in DNA barcoding. Front Ecol Evol 7:302. https://doi.org/10.3389/fevo.2019.00302
Ebert DA, Dando M, Fowler S (2021) Sharks of the world: a complete guide. Princeton University Press, Princeton, NJ
Feldheim KA, Gruber SH, Ashley MV (2002) The breeding biology of lemon sharks at a tropical nursery lagoon. Proc R Soc Lond Ser B 269:1655–1661. https://doi.org/10.1098/rspb.2002.2051
Grogan ED, Lund R (2004) The origin and relationships of early chondrichthyes. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Baton Rouge, pp 3–32
Gruber SH, Nelson DR, Morrissey JF (1988) Patterns of activity and space utilization of lemon sharks, Negaprion brevirostris, in a shallow Bahamian lagoon. Bull Mar Sci 43:61–76
Hamlett WC, Koob TJ (1999) Female reproductive system. In: Hamlett WC (ed) Sharks, skates, and rays: the biology of elasmobranch fishes. John Hopkins University Press, Baltimore, pp 398–443
Hamlett WC, Knight DP, Pereira FTV, Steele J, Sever DM (2005a) Oviducal glands in chondrichthyans. In: Hamlett WC (ed) Reproductive biology and phylogeny of Chondrichthyes: sharks, Batoids and chimaeras. Science Publishers, Enfield, pp 301–335
Hamlett WC, Kormanik G, Storrie M, Stevens B, Walker TI (2005b) Chondrichthyan parity, lecithotrophy and matrotrophy. In: Hamlett WC (ed) Reproductive biology and phylogeny of chondrichthyes: sharks, batoids and chimaeras. Science Publishers Inc, Enfield, pp 395–434
Hart PJB, Reynolds JD (2002) Handbook of fish biology and fisheries: Volume 2 Fisheries. Blackwell, London
Heithaus MR (2001) The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet and seasonal changes in catch rates. Environ Biol Fish 61:25–36. https://doi.org/10.1023/A:1011021210685
Henderson AC (2020) A review of potential taxonomic barriers to the effective management of gulf elasmobranch fisheries. Aquat Ecosyst Health Manage 23:210–219. https://doi.org/10.1080/14634988.2020.1800327
Henderson AC, Reeve AJ (2014) Assessment of shark population movements, delineations and breeding grounds in the Sultanate of Oman. Ministry of Agriculture and Fisheries, Muscat, p 63
Henderson AC, Al-Oufi H, McIlwain JL (2008) Survey, status and utilization of the Elasmobranch Fishery Resources of the Sultanate of Oman. Ministry of Agriculture and Fisheries, Muscat, p 136
Henderson AC, Reeve AJ, Jabado RW, Naylor GJP (2016) Taxonomic assessment of sharks, rays and guitarfishes (Chondrichthyes: Elasmobranchii) from South-Eastern Arabia, using the NADH dehydrogenase subunit 2 (NADH2) gene. Zool J Linnean Soc 176:399–442. https://doi.org/10.1111/zoj.12309
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. https://doi.org/10.3354/meps337287
Jabado RW (2018) The fate of the most threatened order of elasmobranchs: shark-like batoids (Rhinopristiformes) in the Arabian Sea and adjacent waters. Fish Res 204:448–457. https://doi.org/10.1016/j.fishres.2018.03.022
Jabado RW, Spaet JLY (2017) Elasmobranch fisheries in the Arabian seas region: characteristics, trade and management. Fish Fish 18:1096–1118. https://doi.org/10.1111/faf.12227
Jabado RW, Al Ghais SM, Hamsa W, Henderson AC, Ahmad MA (2013) First record of the sand tiger shark, Carcharias taurus, from United Arab Emirates waters. Mar Biodivers Rec 6:e27. https://doi.org/10.1017/S1755267213000043
Jabado RW, Al Ghais SM, Hamza W, Henderson AC (2014a) The shark fishery in The United Arab Emirates: an interview based approach to assess the status of sharks. Aquat Conserv Mar Freshwat Ecosyst 25:800–816. https://doi.org/10.1002/aqc.2477
Jabado RW, Al Ghais SM, Hamza W, Shivji MS, Henderson AC (2014b) Shark diversity in the Arabian/Persian Gulf higher than previously thought: insights based on species composition of shark landings in the United Arab Emirates. Mar Biodivers 45:719–731. https://doi.org/10.1007/s12526-014-0275-7
Jabado RW, Al Hameli SM, Grandcourt EM, Al Dhaheri SS (2018) Low abundance of sharks and rays in baited remote underwater video surveys in the Arabian Gulf. Sci Rep 8:15597. https://doi.org/10.1038/s41598-018-33611-8
Jabado RW, Ebert DA, Al Dhaheri SS (2022) Resolution of the Aetomylaeus nichofii species complex, with the description of a new eagle ray species from the Northwest Indian Ocean and a key to the genus Aetomylaeus (Myliobatiformes: Myliobatidae). Mar Biodivers 52:1–13. https://doi.org/10.1007/s12526-021-01234-4
Johnson MS, Kraver DW, Renshaw GM, Rummer JL (2016) Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv Physiol 4:1–11. https://doi.org/10.1093/conphys/cow003
Kessel ST, Gruber SH, Gledhill KS, Bond ME, Perkins RG (2013) Aerial survey as a tool to estimate abundance and describe distribution of a carcharhinid species, the lemon shark, Negaprion brevirostris. J Mar Biol 2013:1–10. https://doi.org/10.1155/2013/597383
Klimley AP (2013) The biology of sharks and rays. The University of Chicago Press, Chicago
Last PR, Naylor GJP, Séret B, White WT, de Carvalho MR, Stehmann MFW (2016) Rays of the world. CSIRO Publishing, Clayton South
Lucifora LO, de Carvalho MR, Kyne PM, White WT (2015) Freshwater sharks and rays. Curr Biol 25:R971–R973. https://doi.org/10.1016/j.cub.2015.09.004
Meyer W, Seegers U (2012) Basics of skin structure and function in elasmobranchs: a review. J Fish Biol 80:1940–1967. https://doi.org/10.1111/j.1095-8649.2011.03207.x
Moore ABM (2012) Elasmobranchs of the Persian (Arabian) Gulf: ecology, human aspects and research priorities for their improved management. Rev Fish Biol 22:35–61. https://doi.org/10.1007/s11160-011-9222-x
Moore ABM (2015) A review of sawfishes (Pristidae) in the Arabian region: diversity, distribution, and functional extinction of large and historically abundant marine vertebrates. Aquat Conserv Mar Freshwat Ecosyst 25:656–677. https://doi.org/10.1002/aqc.2441
Moore ABM, McCarthy ID, Carvalho GR, Peirce R (2012a) Species, sex, size and male maturity composition of previously unreported elasmobranch landings in Kuwait, Qatar and Abu Dhabi Emirate. J Fish Biol 80:1619–1642. https://doi.org/10.1111/j.1095-8649.2011.03210.x
Moore ABM, Ward RD, Peirce R (2012b) Sharks of the Persian (Arabian) Gulf: a first annotated checklist (Chondrichthyes: Elasmobranchii). Zootaxa 3167:1–16. https://doi.org/10.11646/zootaxa.3167.1.1
Moore AB, Almojil D, Harris M, Jabado RW, White WT (2013) New biological data on the rare, threatened shark Carcharhinus leiodon (Carcharhinidae) from the Persian Gulf and Arabian Sea. Mar Freshw Res 65:327–332. https://doi.org/10.1071/MF13160
Morrissey JF, Gruber SH (1993) Home range of juvenile lemon sharks, Negaprion brevirostris. Copeia 1993:425–434. https://doi.org/10.2307/1447141
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850. https://doi.org/10.1126/science.113865
Naser HA (2013) Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: a review. Mar Pollut Bull 72:6–13. https://doi.org/10.1016/j.marpolbul.2013.04.030
Naylor GJP, Ryburn JA, Fedrigo O, López JA (2005) Phylogenetic relationships among the major lineages of modern elasmobranchs. In: Hamlett WC (ed) Reproductive biology and phylogeny of chondrichthyes. Science Publishers, Enfield, pp 1–26
Naylor GJP, Caira JN, Jensen K, Rosana KAM, White WT, Last PR (2012) A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull Am Mus Nat Hist 367:1–262
Osgood GJ, Baum JK (2015) Reef sharks: recent advances in ecological understanding to inform conservation. J Fish Biol 87:1489–1523. https://doi.org/10.1111/jfb.12839
Osgood GJ, White ER, Baum JK (2021) Effects of climate-change-driven gradual and acute temperature changes on shark and ray species. J Anim Ecol 90:2547–2559. https://doi.org/10.1111/1365-2656.13560
Paerl HW (2006) Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: interactive effects of human and climatic perturbations. Ecol Eng 26:40–54. https://doi.org/10.1016/j.ecoleng.2005.09.006
Palumbi SR, Sandifer PA, Allan JD, Beck MW, Fautin DG, Fogarty MJ, Halpern BS, Incze LS, Leong JA, Norse E, Stachowicz JJ, Wall DH (2009) Managing for ocean biodiversity to sustain marine ecosystem services. Front Ecol Environ 7:204–211. https://doi.org/10.1890/070135
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F (1998) Fishing down marine food webs. Science 279:860–863. https://doi.org/10.1126/science.279.5352.860
Pratt HL (1993) The storage of spermatozoa in the oviducal glands of western North Atlantic sharks. Environ Biol Fish 38:139–149. https://doi.org/10.1007/978-94-017-3450-9_12
Pratt HL Jr, Carrier JC (2011) Elasmobranch courtship and mating behavior. In: Hamlett WC (ed) Reproductive biology and phylogeny of chondrichthyes: sharks, batoids and chimaeras. CRC Press, Baton Rouge, pp 139–175
Robinson DP, Braverstock W, Al-Jura A, Hyland K, Khazanehdari KA (2011) Annually recurring parthenogenesis in a zebra shark Stegostoma fasciatum. J Fish Biol 79:1376–1382. https://doi.org/10.1111/j.1095-8649.2011.03110.x
Robinson DP, Jaidah MY, Bach SS, Rohner CA, Jabado RW, Ormond R, Pierce SJ (2017) Some like it hot: repeat migration and residency of whale sharks within an extreme natural environment. PLoS One 12:e0185360. https://doi.org/10.1371/journal.pone.0185360
Savolainen V, Cowan RS, Vogler AP, Roderick GK, Lane R (2005) Towards writing the encyclopaedia of life: an introduction to DNA barcoding. Philos Trans R Soc B Biol Sci 360:1805–1811. https://doi.org/10.1098/rstb.2005.1730
Sievers M, Brown CJ, Tulloch VJD, Pearson RM, Haig JA, Turschwell MP, Connolly RM (2019) The role of vegetated coastal wetlands for marine megafauna conservation. Trends Ecol Evol 34:807–817. https://doi.org/10.1016/j.tree.2019.04.004
Spaet JLY, Jabado RW, Henderson AC, Moore ABM, Berumen ML (2015) Population genetics of four heavily exploited shark species around the Arabian peninsula. Ecol Evol 5:2317–2332. https://doi.org/10.1002/ece3.1515
Terborgh J, Holt RD, Estes JA (2010) Trophic cascades: what they are, how they work, and why they matter. In: Terborgh J, Estes JA (eds) Trophic cascades: predators, prey, and the changing dynamics of nature. Island Press, Washington, pp 1–18
Thorson TB (1971) Movement of bull sharks, Carcharhinus leucas, between Caribbean Sea and Lake Nicaragua demonstrated by tagging. Copeia 1971:336–338. https://doi.org/10.2307/1442846
Wallach AD, Izhaki I, Toms JD, Ripple WJ, Shanas U (2015) What is an apex predator? Oikos 124:1453–1461. https://doi.org/10.1111/oik.01977
Wetherbee BM, Cortés E (2004) Food consumption and feeding habits. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Baton Rouge, pp 225–246
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Supplementary Data
(DOCX 32 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Henderson, A.C., Al Hameli, S. (2024). Sharks and Rays of the United Arab Emirates. In: Burt, J.A. (eds) A Natural History of the Emirates. Springer, Cham. https://doi.org/10.1007/978-3-031-37397-8_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-37397-8_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37396-1
Online ISBN: 978-3-031-37397-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)